Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom
Abstract
:1. Introduction
2. Results and Discussion
2.1. Caffeic Acid, Chlorogenic Acid, and Quercetin Do Not Protect against C. d. terrificus Venom-Induced Neuromuscular Blockade In Vitro
2.2. Systemic Effects
2.3. Analysis of the Tannic Acid–Venom Interaction
2.4. Other Parameters Analyzed
3. Further Considerations and Conclusions
4. Material and Methods
4.1. Phytochemicals
4.2. Venom
4.3. Antivenom
4.4. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Animals
4.6. Mouse Phrenic Nerve-Diaphragm Preparations
4.7. In Vivo Experiments
4.7.1. Selection of C. d. terrificus Venom Dose for Severe Envenomation
4.7.2. The Efficacy of Tannic Acid against the Lethality of C. d. terrificus Venom
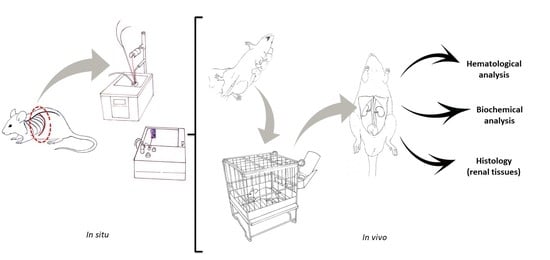
4.7.3. Analytical Procedures Performed on Blood and Renal Tissue Samples
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frare, B.T.; Silva Resende, Y.K.; Dornelas, B.C.; Jorge, M.T.; Souza Ricarte, V.A.; Alves, L.M.; Izidoro, L.F.M. Clinical, laboratory, and therapeutic aspects of Crotalus durissus (South American rattlesnake) victims: A literature review. BioMed Res. Int. 2019, 2019, 1345923. [Google Scholar] [CrossRef]
- Bucaretchi, F.; De Capitani, E.M.; Branco, M.M.; Fernandes, L.C.; Hyslop, S. Coagulopathy as the main systemic manifestation after envenoming by a juvenile South American rattlesnake (Crotalus durissus terrificus): Case report. Clin. Toxicol. 2013, 51, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Marques, M.M.; Cupo, P.; Coimbra, T.M.; Hering, S.E.; Rossi, M.A.; Laure, C.J. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon 1985, 23, 631–636. [Google Scholar] [CrossRef]
- Warrell, D.A. Snakebites in Central and South America: Epidemiology, clinical features and clinical management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Comstock Publishers/Cornell University Press: Ithaca, NY, USA, 2004; Volume 2, pp. 709–761. [Google Scholar]
- Amaral, C.F.S.; Rezende, N.A.; Pedrosa, T.M.G.; da Silva, O.A.; Pedroso, E.R.P. Afibrinogenemia secundária a acidente ofídico crotálico (Crotalus durissus terrificus). Rev. Inst. Med. Trop São Paulo 1988, 30, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Pinho, F.M.; Zanetta, D.M.; Burdmann, E.A. Acute renal failure after Crotalus durissus snakebite: A prospective survey on 100 patients. Kidney Int. 2005, 67, 659–667. [Google Scholar] [CrossRef]
- Sano-Martins, I.S.; Tomy, S.C.; Campolina, D.; Dias, M.B.; de Castro, S.C.; de Souza e Silva, M.C.; Amaral, F.C.; Rezende, N.A.; Kamiguti, A.S.; Warrell, D.A.; et al. Coagulopathy following lethal and non-lethal envenoming of humans by the South American rattlesnake (Crotalus durissus) in Brazil. Q. J. Med. 2001, 94, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.E.; Campanholi, J.; Cavalcante, R.L.; Moreno, F.S.; Yoshida, E.H.; Dini, M.M.J.; Aranha, E.F.C.; Cogo, J.C.; Dias, L.; Hyslop, S.; et al. Experimental model for removal of snake venom via hemoperfusion in rats. J. Vet. Emerg. Crit. Care 2020, 30, 286–294. [Google Scholar] [CrossRef]
- WHO World Health Organization. Available online: https://www.who.int/snakebites/antivenoms/en/ (accessed on 1 May 2021).
- Gutiérrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and methyl varespladib (LY333013) abrogate or delay lethality induced by presynaptically acting neurotoxic snake venoms. Toxins 2020, 12, 131. [Google Scholar] [CrossRef]
- Cesar, P.H.S.; Trento, M.V.; Sales, T.A.; Simão, A.C.; Ramalho, T.; Marcussi, S. Vanillic acid as phospholipase A2 and proteases inhibitor: In vitro and computational analyses. Biotechnol. Appl. Biochem. 2020, 68, 486–496. [Google Scholar] [CrossRef]
- Vaz de Melo, P.D.; de Almeida Lima, S.; Araújo, P.; Medina Santos, R.; Gonzalez, E.; Alves Belo, A.; Machado-de-Ávila, R.A.; Costal-Oliveira, F.T.; Soccol, V.; Guerra-Duarte, C.; et al. Immunoprotection against lethal effects of Crotalus durissus snake venom elicited by synthetic epitopes trapped in liposomes. Int. J. Biol. Macromol. 2020, 161, 299–307. [Google Scholar] [CrossRef]
- Marques, T.R.; Braga, M.A.; Cesar, P.H.S.; Marcussi, S.; Corrêa, A.D. Jabuticaba (Plinia jaboticaba) skin extracts as inhibitors of phospholipases A2 and proteases. An. Acad. Bras. Cienc. 2019, 91, e20180248. [Google Scholar] [CrossRef]
- Teixeira, M.L.; Marcussi, S.; Rezende, D.A.C.S.; Magalhães, M.L.; Nelson, D.L.; Cardoso, M.G. Essential oil from Lippia origanoides (Verbenaceae): Haemostasis and enzymes activity alterations. Med. Chem. 2019, 15, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.B.; Ferreira, M.J.P.; Belchor, M.N.; Costa, C.R.C.; Novaes, D.P.; dos Santos Junior, A.B.; Tamayose, C.I.; Pinho, M.V.T.; de Oliveira, M.A.; Toyama, M.H. Evaluation of the inhibitory potential of casuarictin, an ellagitannin isolated from white mangrove (Laguncularia racemosa) leaves, on snake venom secretory phospholipase A2. Mar. Drugs 2019, 17, 403. [Google Scholar] [CrossRef]
- Tamayose, C.I.; Romoff, P.; Toyama, D.O.; Gaeta, H.H.; Costa, C.R.C.; Belchor, M.N.; Ortolan, B.D.; Velozo, L.S.M.; Kaplan, M.A.C.; Ferreira, M.J.P.; et al. Non-clinical studies for evaluation of 8-C-rhamnosyl apigenin purified from Peperomia obtusifolia against acute edema. Int. J. Mol. Sci. 2017, 18, 1972. [Google Scholar] [CrossRef]
- Toyama, D.O.; Ferreira, M.J.; Romoff, P.; Fávero, A.O.; Gaeta, H.H.; Toyama, M.H. Effect of chlorogenic acid (5-caffeoylquinic acid) isolated from Baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, 2014, 726585. [Google Scholar] [CrossRef]
- Barone, J.M.; Frezzatti, R.; Silveira, P.F. Effects of N-acetyl-L-cysteine on redox status and markers of renal function in mice inoculated with Bothrops jararaca and Crotalus durissus terrificus venoms. Toxicon 2014, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frezzatti, R.; Silveira, P.F. Allopurinol reduces the lethality associated with acute renal failure induced by Crotalus durissus terrificus snake venom: Comparison with probenecid. PLoS Negl. Trop. Dis. 2011, 5, e1312. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.A.; de Oliveira, S.C.; Diz Filho, E.B.; Fonseca, F.V.; Baldissera, L., Jr.; Antunes, E.; Ximenes, R.M.; Monteiro, H.S.; Rabello, M.M.; Hernandes, M.Z.; et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chem. Biol. Interact. 2011, 189, 9–16. [Google Scholar] [CrossRef]
- de Jesus Reis Rosa, L.; Silva, G.A.; Filho, J.A.; Silva, M.G.; Cogo, J.C.; Groppo, F.C.; Oshima-Franco, Y. The inhibitory effect of Camellia sinensis extracts against the neuromuscular blockade of Crotalus durissus terrificus venom. J. Venom. Res. 2010, 1, 1–7. [Google Scholar]
- de Sousa Alegre, V.; Barone, J.M.; Yamasaki, S.C.; Zambotti-Villela, L.; Silveira, P.F. Lipoic acid effects on renal function, aminopeptidase activities and oxidative stress in Crotalus durissus terrificus envenomation in mice. Toxicon 2010, 56, 402–410. [Google Scholar] [CrossRef]
- Maiorano, V.A.; Marcussi, S.; Daher, M.A.; Oliveira, C.Z.; Couto, L.B.; Gomes, A.O.; França, S.C.; Soares, A.M.; Pereira, P.S. Antiophidian properties of the aqueous extract of Mikania glomerata. J. Ethnopharmacol. 2005, 102, 364–370. [Google Scholar] [CrossRef]
- Floriano, R.S.; Nogueira, R.M.; Sakate, M.; Laposy, C.B.; da Motta, Y.P.; Sangiorgio, F.; David, H.C.; Nabas, J.M. Effect of Mikania glomerata (Asteraceae) leaf extract combined with anti-venom serum on experimental Crotalus durissus (Squamata: Viperidae) envenomation in rats. Rev. Biol. Trop. 2009, 57, 929–937. [Google Scholar] [CrossRef]
- Diogo, L.C.; Fernandes, R.S.; Marcussi, S.; Menaldo, D.L.; Roberto, P.G.; Matrangulo, P.V.; Pereira, O.S.; França, S.C.; Giuliatti, S.; Soares, A.M.; et al. Inhibition of snake venoms and phospholipases A2 by extracts from native and genetically modified Eclipta alba: Isolation of active coumestans. Basic Clin. Pharmacol. Toxicol. 2009, 104, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Farrapo, N.M.; Rocha Junior, D.S.; Silva, M.G.; Cogo, J.C.; Dal Belo, C.A.; Rodrigues-Simioni, L.; Groppo, F.C.; Oshima-Franco, Y. Antiophidian mechanisms of medicinal plants. In Flavonoids: Biosynthesis, Biological Effects and Dietary Sources; (Nutrition and Diet Research Progress Series); Keller, R.B., Ed.; Nova Science Publishers Inc: New York, NY, USA, 2009; pp. 249–262. Available online: https://www.researchgate.net/publication/287573650_Antiophidian_mechanisms_of_medicinal_plants (accessed on 31 August 2021).
- Mendes, M.M.; Oliveira, C.F.; Lopes, D.S.; Vale, L.H.; Alcântara, T.M.; Izidoro, L.F.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Soares, A.M.; Rodrigues, V.M. Anti-snake venom properties of Schizolobium parahyba (Caesalpinoideae) aqueous leaves extract. Phytother. Res. 2008, 22, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Batina, M.F.; Cintra, A.C.; Veronese, E.L.; Lavrador, M.A.; Giglio, J.R.; Pereira, O.S.; Dias, D.A.; França, S.C.; Sampaio, S.V. Inhibition of the lethal and myotoxic activities of Crotalus durissus terrificus venom by Tabernaemontana catharinensis: Identification of one of the active components. Planta Med. 2000, 66, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.C.; Toyama, M.; Marangoni, S.; Oliveira, B.; Cirino, G.; Antunes, E.; de Nucci, G. Effect of crotapotin and heparin on the rat paw oedema induced by different secretory phospholipases A2. Toxicon 2000, 38, 199–208. [Google Scholar] [CrossRef]
- Freitas, T.V.; Frézard, F. Encapsulation of native crotoxin in liposomes: A safe approach for the production of antivenom and vaccination against Crotalus durissus terrificus venom. Toxicon 1997, 35, 91–100. [Google Scholar] [CrossRef]
- Vancetto, M.D.; Curi, L.C.; Pereira, C.A. Neutralization of the effect of Crotalus durissus terrificus venom by gangliosides. Braz J. Med. Biol Res. 1995, 28, 553–556. [Google Scholar]
- Farrapo, N.M.; Silva, G.A.A.; Costa, K.N.; Silva, M.G.; Cogo, J.C.; Dal Belo, C.A.; Dos Santos, M.G.; Groppo, F.C.; Oshima-Franco, Y. Inhibition of Bothrops jararacussu venom activities by Plathymenia reticulata Benth extracts. J. Venom. Res. 2011, 2, 52–58. [Google Scholar]
- Della Torre, A.; Albuquerque, L.B.L.; Farrapo, N.M.; Oshima-Franco, Y.; Santos, M.G.; Tavares, R.V.S.; Rodas, A.C.D.; Dal Belo, C.A.; Cardoso, C.R.P.; Varanda, E.A.; et al. Mutagenicity induced by the hydroalcoholic extract of the medicinal plant Plathymenia reticulata Benth. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 190–198. [Google Scholar] [CrossRef]
- Collaço, R.C.; Cogo, J.C.; Rodrigues-Simioni, L.; Rocha, T.; Oshima-Franco, Y.; Randazzo-Moura, P. Protection by Mikania laevigata (guaco) extract against the toxicity of Philodryas olfersii snake venom. Toxicon 2012, 60, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Novaes, L.F.; Baldi da Silva, E.L.; Santana, M.N.; Silva, M.G.; Soares-Silva, J.O.; Lima, S.L.T.; Gerenutti, M.; Oshima-Franco, Y. The contribution of solubilizers to Mikania laevigata extracts pharmacological effects: A traditional bronchodilator plant. OA Altern. Med. 2014, 2, 11–18. [Google Scholar]
- Oshima-Franco, Y.; Dal Belo, C.A. Recognizing antiophidian plants using the neuromuscular junction apparatus. Int. J. Complement. Altern. Med. 2017, 5, 165. [Google Scholar]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á.; Vargas, M.; Villalta, M.; Sánchez, A.; Sanz, L.; Lomonte, B.; et al. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: State-of-the-art and challenges ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Hodgson, W.C.; Isbister, G.K. Antivenom for neuromuscular paralysis resulting from snake envenoming. Toxins 2017, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the therapeutics of anti-snake venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef]
- Pithayanukul, P.; Leanpolchareanchai, J.; Bavovada, R. Inhibitory effect of tea polyphenols on local tissue damage induced by snake venoms. Phytother. Res. 2010, 24, S56–S62. [Google Scholar] [CrossRef]
- Homma, M.; Abe, R.; Okonogi, T.; Kosuge, T.; Mishima, S. Studies on Habu snake and Erabu sea snake venoms outlines of biological toxicities of the two snake venoms, and inhibitory actions of tannic acid on them. Nihon Ishigaku Zasshi. 1965, 20, 281–289. (In Japanese) [Google Scholar]
- Pithayanukul, P.; Ruenraroengsak, P.; Bavovada, R.; Pakmanee, N.; Suttisri, R. In vitro investigation of the protective effects of tannic acid against the activities of Naja kaouthia venom. Pharm. Biol. 2007, 45, 94–97. [Google Scholar] [CrossRef]
- Sia, F.Y.; Vejayan, J.; Jamuna, A.; Ambu, S. Efficacy of tannins from Mimosa pudica and tannic acid in neutralizing cobra (Naja kaouthia) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 42–48. [Google Scholar] [CrossRef]
- de Moura, V.M.; da Costa Guimarães, N.; Batista, L.T.; Freitas-de-Sousa, L.A.; de Sousa Martins, J.; de Souza, M.C.S.; Oliveira de Almeida, P.D.; Monteiro, W.M.; de Oliveira, R.B.; Dos-Santos, M.C.; et al. Assessment of the anti-snakebite properties of extracts of Aniba fragrans Ducke (Lauraceae) used in folk medicine as complementary treatment in cases of envenomation by Bothrops atrox. J. Ethnopharmacol. 2018, 213, 350–358. [Google Scholar] [CrossRef]
- Okonogi, T.; Hattori, A.; Ogiso, A.; Mitsui, S. Detoxification by persimmon tannin of snake venoms and bacterial toxins. Toxicon 1979, 17, 524–527. [Google Scholar] [CrossRef]
- Yugarani, T.; Tan, B.K.; Das, N.P. The effects of tannic acid on serum lipid parameters and tissue lipid peroxides in the spontaneously hypertensive and Wistar Kyoto rats. Planta Med. 1993, 59, 28–31. [Google Scholar] [CrossRef]
- Xue, Y.; Li, M.; Xue, Y.; Jin, W.; Han, X.; Zhan, J.; Chu, X.; Li, Z.; Chu, L. Mechanisms underlying the protective effect of tannic acid against arsenic trioxide-induced cardiotoxicity in rats: Potential involvement of mitochondrial apoptosis. Mol. Med. Rep. 2020, 22, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Claassen, V. Intraperitoneal drug administration. In Neglected Factors in Pharmacology and Neuroscience Research: Biopharmaceutics, Animal Characteristics Maintenance, Testing Conditions; (Techniques in the Behavioral and Neural Sciences Series); Claassen, V., Ed.; Elsevier: Gainesville, FL, USA, 1994; Volume 12, pp. 46–58. [Google Scholar]
- Smith, G.S.; Walter, G.L.; Walker, R.M. Clinical pathology in non-clinical toxicology testing. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: New York, NY, USA, 2013; pp. 565–594. [Google Scholar]
- Gorriz, J.L.; Martinez-Castelão, A. Proteinuria: Detection and role in native renal disease progression. Transplant. Rev. 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Deckert, T.; Feldt-Rasmussen, B.; Borch-Johnsen, K.; Jensen, T.; Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989, 32, 219–226. [Google Scholar] [CrossRef]
- Perkovic, V.; Verdon, C.; Ninomiya, T.; Barzi, F.; Cass, A.; Patel, A.; Jardine, M.; Gallagher, M.; Turnbull, F.; Chalmers, J.; et al. The relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoS Med. 2008, 5, e207. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.H.; Cardoso, F.F.; Cavalcante, W.G.L.; Soares, A.M.; Dal-Pai, M.; Gallacci, M.; Fontes, M.R.M. Structural basis for the inhibition of a phospholipase A2-like toxin by caffeic and aristolochic acids. PLoS ONE 2015, 10, e0133370. [Google Scholar]
- dos Santos, J.I.; Cardoso, F.F.; Soares, A.M.; Dal Pai Silva, M.; Gallacci, M.; Fontes, M.R. Structural and functional studies of a bothropic myotoxin complexed to rosmarinic acid: New insights into Lys49-PLA2 inhibition. PLoS ONE 2011, 6, e28521. [Google Scholar] [CrossRef]
- Nirmal, M.; Om Praba, G.; Verlmurugan, D. Modeling studies on phospholipase A2-inhibitor complexes. Indian J. Biochem. Biophys. 2008, 45, 256–262. [Google Scholar] [PubMed]
- Tribuiani, N.; da Silva, A.M.; Ferraz, M.C.; Silva, M.G.; Bentes, A.P.; Graziano, T.S.; dos Santos, M.G.; Cogo, J.C.; Varanda, E.A.; Groppo, F.C.; et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement. Altern Med. 2014, 14, 48. [Google Scholar]
- Chiou, Y.L.; Lin, S.R.; Hu, W.P.; Chang, L.S. Quercetin modulates activities of Taiwan cobra phospholipase A2 via its effects on membrane structure and membrane-bound mode of phospholipase A2. J. Biosci. 2012, 37, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Belchor, M.N.; Gaeta, H.H.; Rodrigues, C.F.B.; da Cruz Costa, C.R.; Toyama, D.O.; Passero, L.F.D.; Laurenti, M.D.; Toyama, M.H. Evaluation of rhamnetin as an inhibitor of the pharmacological effect of secretory phospholipase A2. Molecules 2017, 22, 1441. [Google Scholar] [CrossRef] [PubMed]
- Toyama, D.O.; Gaeta, H.H.; de Pinho, M.V.; Ferreira, M.J.; Romoff, P.; Matioli, F.F.; Magro, A.J.; Fontes, M.R.; Toyama, M.H. An evaluation of 3-rhamnosylquercetin, a glycosylated form of quercetin, against the myotoxic and edematogenic effects of sPLA2 from Crotalus durissus terrificus. Biomed. Res. Int. 2014, 2014, 341270. [Google Scholar] [CrossRef] [PubMed]
- Dal Belo, C.A.; Lucho, A.P.; Vinadé, L.; Rocha, L.; França, H.S.; Marangoni, S.; Rodrigues-Simioni, L. In vitro antiophidian mechanisms of Hypericum brasiliense Choisy standardized extract: Quercetin-dependent neuroprotection. Biomed. Res. Int. 2013, 2013, 943520. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Das, N.P. Protective effects of tannic acid and related natural compounds on Crotalus adamenteus subcutaneous poisoning in mice. Pharmacol. Toxicol. 1993, 72, 290–295. [Google Scholar] [CrossRef]
- Shabbir, A.; Shahzad, M.; Masci, P.; Gobe, G.C. Protective activity of medicinal plants and their isolated compounds against the toxic effects from the venom of Naja (cobra) species. J. Ethnopharmacol. 2014, 157, 222–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.M. The detoxifying effects of structural elements of persimmon tannin on Chinese cobra phospholipase A2 correlated with their structural disturbing effects well. J. Food Drug Anal. 2017, 25, 731–740. [Google Scholar] [CrossRef]
- Ferreira-Rodrigues, S.C.; Yoshida, E.H.; Oshima-Franco, Y.; Santos, M.G.; Seibert, C.S. Neuromuscular block induced by Bothrops moojeni snake venom and the effect of Jatropha elliptica starch. Rev. Ibero-Am. Ciênc. Ambient. 2021, 12, 651–662. [Google Scholar]
- National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington DC, USA, 2011. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Bülbring, E. Observation on the isolated phrenic nerve diaphragm preparation of the rat. Br. J. Pharmacol. 2007, 120, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Celestino Parrilha, L.A.; Duarte Moraes, M.; Amaral Filho, J.; Cogo, J.C.; dos Santos, M.G.; Franco, L.M.; Groppo, F.C.; Puebla, P.; San Feliciano, A.; et al. The effect of lupane triterpenoids (Dipteryx alata Vogel) in the in vitro neuromuscular blockade and myotoxicity of two snake venoms. Curr. Org. Chem. 2012, 16, 2717–2723. [Google Scholar] [CrossRef]
- Ferraz, M.C.; Yoshida, E.H.; Tavares, R.V.; Cogo, J.C.; Cintra, A.C.; Dal Belo, C.A.; Franco, L.M.; dos Santos, M.G.; Resende, F.A.; Varanda, E.A.; et al. An isoflavone from Dipteryx alata Vogel is active against the in vitro neuromuscular paralysis of Bothrops jararacussu snake venom and bothropstoxin-I and prevents venom-induced myonecrosis. Molecules 2014, 19, 5790–5805. [Google Scholar] [CrossRef] [PubMed]
- Boer-Lima, P.A.; Gontijo, J.A.; Cruz-Höfling, M.A. Histologic and functional renal alterations caused by Bothrops moojeni snake venom in rats. Am. J. Trop. Med. Hyg. 1999, 61, 698–706. [Google Scholar] [CrossRef]
- Yoshida, E.H.; Dini, M.M.J.; Campanholi, J.; Cogo, J.C.; Grotto, D.; Hyslop, S.; Hanai-Yoshida, V.M.; Oshima-Franco, Y. Acute kidney injury caused by the intraperitoneal injection of Bothrops jararaca venom in rats. Nat. Prod. Res. 2020, 34, 2533–2538. [Google Scholar] [CrossRef] [PubMed]






| Substance(s) | Activity Evaluated | Main Findings | Reference |
|---|---|---|---|
| Varespladib (LY315920) and its orally bioavailable prodrug, methyl-varespladib (LY333013) | Protection against the lethality of neurotoxic snake venoms (Notechis scutatus, Crotalus durissus terrificus, Bungarus multicinctus, Oxyuranus scutellatus) in mice. | Varespladib abrogated or delayed the neurotoxic manifestations induced by some venoms in which neurotoxicity was mainly dependent on presynaptically active PLA2s. LY315920 reversed the paralytic manifestations in severely envenomed mice. | [10] |
| Vanillic acid | Inhibition of PLA2 and proteases. | Vanillic acid inhibited the PLA2 activity of Bothrops alternatus (∼25% inhibition) and the caseinolytic activity of Bothrops atrox (∼30%), Bothrops jararacussu (∼44%), and C. d. terrificus (∼33%). | [11] |
| Antibodies against synthetic peptides | Antigenicity/immunogenicity of crotoxin and crotamine. | Antibodies against synthetic peptides protected mice against venom lethality. | [12] |
| Aqueous and methanolic extracts of Plinia jaboticaba skins | Inhibition of PLA2 and proteases. | Inhibition of the PLA2 activity of Bothrops moojeni and Crotalus durissus terrificus venoms, but not B. atrox venom. The greatest inhibition of hemolysis was observed for the methanolic extract when incubated with B. moojeni and C. d. terrificus venoms (inhibition of 21–100%). Thrombolysis induced by B. moojeni and C. d. terrificus venoms was inhibited by both extracts (by 32–83% and 51–83% for the aqueous and methanolic extracts, respectively). | [13] |
| Essential oil from Lippia origanoides | Inhibition of PLA2 activity. | Potentiation of hemolytic activity in preincubation protocols and presence of prothrombotic activity. | [14] |
| Casuarictin from Laguncularia racemosa | Inhibition of secretory PLA2 (sPLA2). | The anti-inflammatory activity suggested a potential use of this compound in treating edema and myonecrosis induced by sPLA2. | [15] |
| 8-C-rhamnosyl apigenin from Peperomia obtusifolia | Inhibition of sPLA2 and cytosolic PLA2 (cPLA2). | Inhibition of C. d. terrificus sPLA2 and cPLA2, but also significant inhibition of cyclooxygenase activity. | [16] |
| Chlorogenic acid (5-caffeoylquinic acid, 5CQA), isolated from Baccharis oxyodonta | Effect on sPLA2 structure and pharmacological activity. | 5CQA modulated the inflammatory activity of sPLA2. | [17] |
| N-acetyl-cysteine | Protection against venom-induced renal damage. | The renal protection observed with NAC suggested a potential usefulness, along with antivenom therapy, in envenomation by C. d. terrificus. | [18] |
| Allopurinol and probenecid | Effects of allopurinol and probenecid on venom-induced renal dysfunction. | Allopurinol deserves to be clinically evaluated as an ancillary treatment for snakebite along with antivenom. | [19] |
| Quercetin | Inhibition of sPLA2. | Quercetin inhibited the enzymatic activity and some pharmacological activities of sPLA2, including its antibacterial activity, its ability to induce platelet aggregation, and its myotoxicity, but did not reduce the inflammatory and neurotoxic activities of sPLA2. | [20] |
| Camellia sinensis extract and its metabolites theoflavin and epigallocatechin gallate | Inhibition of venom-induced in vitro neuromuscular blockade. | The extract and theoflavin, but not epigallocatechin gallate, protected against irreversible neuromuscular blockade induced by C. d. terrificus venom in mouse phrenic-nerve diaphragm. | [21] |
| Lipoic acid | Effects of lipoic acid (LA) on lethality, renal dysfunction, aminopeptidase and GSSG/GSH levels in venom-injected mice. | LA solubilized/removed proteins from the membrane-bound fraction with impairment of most aminopeptidase activity but could still be useful for the treatment of directly induced venom nephrotoxicity. | [22] |
| Aqueous extract from Mikania glomerata | Inhibition of PLA2s, metalloproteinases and serine proteinases. | PLA2 activity and C. d. terrificus venom-induced edema were inhibited around 100% and ∼40%, respectively. Total inhibition of clotting activity. | [23] |
| Attenuation of clinical and laboratory manifestations of venom in Wistar rats. | Envenomation caused hypothermia, local edema, sedation, and a decrease in locomotion. The extract enhanced the recovery from sedation. | [24] | |
| Genetically modified Eclipta alba and active coumestans | Inhibition of PLA2 and venom-induced myotoxicity. | Clone 19 and isolated coumestans (wedelolactone and demethylwedelolactone) inhibited the myotoxic activity of venom PLA2. | [25] |
| Tannic acid | Inhibition of venom-induced in vitro neuromuscular blockade. | Tannic acid abolished the venom-induced paralysis. | [26] |
| Aqueous extract of Schizolobium parahyba (Caesalpinoideae) leaves | Inhibition of PLA2 and biological activities of C. d. terrificus venom. | The aqueous extract of S. parahyba neutralized PLA2 and biological activities (e.g., coagulant activity) of the venom. | [27] |
| Alkaloid from Tabernaemontana catharinensis | Inhibition of venom lethality and myotoxicity. | Tabernaemontana catharinensis could be a useful source for model molecules to neutralize the lethality and myotoxicity of C. d. terrificus venom. | [28] |
| Heparin | The effects of crotapotin (a non-toxic and non-enzymatic acid polypeptide naturally complexed with PLA2 in the venom) and of heparin on rat paw edema induced by different sPLA2. The ability of crotapotin to modulate the enzymatic activity of sPLA2 was also evaluated. | Despite the great homology between the various types of sPLA2, they interacted with crotapotin on cell surfaces in different ways, leading to either inhibition or potentiation of the paw edema by a mechanism unrelated to their enzymatic activity. | [29] |
| Encapsulated crotoxin in liposomes | Assessment of immunogenicity. | Crotoxin encapsulated into dehydration-rehydration vesicles (DRV/crotoxin) was less toxic than crotoxin emulsified in Freund’s complete adjuvant (FCA/crotoxin) and induced lower levels of anti-crotoxin antibodies but similar levels of protection when inoculated at high doses (20 or 70 μg of crotoxin/mouse). When DRV/crotoxin was adsorbed to alum at the time of immunization, it induced antibody and protection levels comparable to those produced by FCA/crotoxin. | [30] |
| Gangliosides | Evaluation of ability of a mixture of gangliosides to neutralize the effects of venom in vitro and in vivo. | Gangliosides effectively neutralized the toxic effects of venom in vitro and in vivo and the intramuscular injection of gangliosides after venom administration protected envenomed animals. | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.C.F.; Yoshida, E.H.; Dini, M.M.J.; Paschoal, A.B.O.; Cogo, J.C.; da Cruz-Höfling, M.A.; Hyslop, S.; Oshima-Franco, Y. Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom. Toxins 2021, 13, 801. https://doi.org/10.3390/toxins13110801
Oliveira ICF, Yoshida EH, Dini MMJ, Paschoal ABO, Cogo JC, da Cruz-Höfling MA, Hyslop S, Oshima-Franco Y. Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom. Toxins. 2021; 13(11):801. https://doi.org/10.3390/toxins13110801
Chicago/Turabian StyleOliveira, Isadora Caruso Fontana, Edson Hideaki Yoshida, Murilo Melo Juste Dini, Ana Beatriz Olívio Paschoal, José Carlos Cogo, Maria Alice da Cruz-Höfling, Stephen Hyslop, and Yoko Oshima-Franco. 2021. "Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom" Toxins 13, no. 11: 801. https://doi.org/10.3390/toxins13110801
APA StyleOliveira, I. C. F., Yoshida, E. H., Dini, M. M. J., Paschoal, A. B. O., Cogo, J. C., da Cruz-Höfling, M. A., Hyslop, S., & Oshima-Franco, Y. (2021). Evaluation of Protection by Caffeic Acid, Chlorogenic Acid, Quercetin and Tannic Acid against the In Vitro Neurotoxicity and In Vivo Lethality of Crotalus durissus terrificus (South American Rattlesnake) Venom. Toxins, 13(11), 801. https://doi.org/10.3390/toxins13110801