1. Introduction
Ligand-gated ion channels are classified into several families of membrane-bound receptors. The Cys-loop family of pentameric receptors is represented widely in the muscle and nervous systems as well as in immune and other cells and plays prominent roles. The Cys-loop family of vertebrates includes nicotinic acetylcholine receptor (nAChR), as well as serotonin type 3, γ-aminobutyric acid (GABA
AR), and glycine receptors. The receptors of this family are characterized by a conserved sequence of 13 amino acid residues confined by two cysteines forming a disulfide bond (Cys loop) in the
N-terminal extracellular domain of each subunit [
1]. Among these receptors, excitatory nAChRs are the targets of numerous natural compounds including toxins from snake venom [
2,
3]. Snake venom neurotoxins of the three-finger toxin (TFT) family efficiently inhibit some nAChR subtypes. For example, α-bungarotoxin (α-Bgt) from krait
Bungarus multicinctus venom binds muscle-type as well as neuronal α7 and α9 nAChRs with nanomolar affinities and blocks ion current through the ion channel of these receptors. α-Cobratoxin (α-Ctx) from cobra
Naja kaouthia venom exhibits similar effects. Based on location and function, nAChRs can be divided into muscle and neuronal ones [
4,
5,
6]. Neuronal nAChRs are localized in the central and peripheral nervous system and are involved in the transmission of fast nerve impulses. Muscle-type nAChRs are located on the postsynaptic membranes of the neuromuscular junction and transmit signals for muscle contraction. Each muscle-type nAChR has two copies of α1 and one each of the β1, δ, and γ subunits (in the embryonic receptor) or ε subunit (in the adult form). In this receptor type, two binding sites for agonists and competitive antagonists are located in the extracellular domain at the interfaces of the α1–δ and α1–γ (or α1–ε) subunits [
5]. Two binding sites have different affinity to acetylcholine, the α–γ site possessing a ∼35–40-fold higher affinity for acetylcholine than the α–δ and α–ε sites [
7]. Some natural toxins, including several α-conotoxins, waglerins, and few TFTs, bind with different affinities to these two binding sites as well. Thus, a short α-neurotoxin from cobra
N. mossambica mossambica (NmmI) was shown to bind with high affinity to α–γ and α–δ subunit interfaces but had a markedly reduced affinity to the α–ε interface [
8]. Furthermore, the mutation of Lys27 to Glu27 in NmmI affected binding at the α–γ site more than the α–δ site [
9] making this mutant selective for α–δ site. The similar results were obtained for two mutants of α-Ctx K23E and K49E, which demonstrated the different affinities to two toxin-binding sites on
Torpedo nAChR with higher and lower affinities at the α–δ and α–γ sites, respectively [
10]. The data for nonconventional TFT, candoxin from
B. candidus also suggest its differential affinity for the α–γ or α–δ sites at the muscle nAChR [
11]. We have found recently that so called αδ-bungarotoxins from
B. candidus venom show different affinity to two binding sites in muscle-type nAChRs, manifesting higher activity at the interface of α–δ subunits [
12].
Until recently α-Bgt and α-Ctx were considered as very specific markers of nAChRs. However, the works of our and other groups [
13,
14,
15] have shown that these toxins inhibit also GABA
AR. Similarly to nAChRs, each GABA
AR is composed of five subunits, including most frequently two α subunits, two β subunits, and one from
γ,
δ,
ε,
θ, or
π subunit. However, contrary to nAChRs which are cation channels, GABA
AR are chloride channels. In mammals, 19 different subunits (six α, three β, three γ, δ, ε, θ, π, and three ρ) are known; they form a wide variety of GABA
A receptor subtypes with distinct subunit composition and unique pharmacological properties [
16]. In the brain, the most abundant GABA
AR isoforms are αβγ and αβδ [
17]. GABA
A αβγ receptors are widely distributed in the brain, while αβδ receptors constitute only a small proportion [
18]. Among α subunits, the most abundant is α1 which is often colocalized with highly expressed β2 and γ2 subunits [
18]. The α2 and α3 subunits are less abundant. Among the β subunits, β2 is most abundant, β3 is reasonably highly expressed, and β1 is least common. Up to 80% of GABA
ARs contain the γ2 subunit [
16]. In the brain, α1β2γ2 is the most common isoform. At present, several clinically used compounds target GABA
AR and still there is a need in finding new molecules for potential design of novel drugs.
Acetylcholine binding proteins (AChBPs), soluble proteins mostly from mollusks, are remarkable structural homologues of the ligand-binding domains of all Cys-loop receptors [
19]. While transmembrane and intracellular motifs are absent in these proteins, the important elements necessary for ligand binding including the C- and F-loops are structurally conserved. This fact made AChBPs and their mutants perfect tools for structural studies on pentameric ligand-gated ion channels. The fact that many studies have revealed some inconsistency in the activity profiles on nAChR and AChBP for a number of ligands was compensated by high-resolution structures of complexes of these ligands with AChBPs. Based on these data, more reliable models of the complexes of the corresponding ligands with the full-length nAChRs were built in order to identify the key amino acid residues and molecular mechanisms that determine the receptor-ligand interactions. In particular, the molecular basis for the high selectivity of α-conotoxin LvIA for α3β2 nAChR [
20] or the difference in affinity of two other α-conotoxins’ analogues to human and rat α7 nAChR [
21] were explained. At present, AChBPs from the mollusks
Aplysia californica and
Lymnaea stagnalis [
22] are widely used, the former is closer pharmacologically to homooligimeric nAChRs while the latter is closer to heterooligomeric receptors.
α-Bgt, being a very efficient blocker of nAChRs, showed fairly weak activity on GABA
AR inhibiting α1β3γ2 receptors by only 19% at 10 µM [
15]. In contrast, α-Ctx inhibited GABA
AR quite effectively manifesting half-maximal inhibitory concentration (IC
50) of 236 nM at α1β3γ receptor [
15]. Some other snake neurotoxins inhibited GABA
AR as well, although not so effectively as α-Ctx. It was suggested that Arg36, present in α-Ctx and being valine in this position of α-Bgt, might be responsible for efficient interaction of α-Ctx with GABA
AR [
6]. In order to test this hypothesis, α-Ctx analogues with or without Arg36 need to be used. Although the most straightforward way would be testing the α-Ctx analogue(s) obtained through site-directed mutagenesis at position occupied by Arg36, there are some problems that should be solved on this way. First one is that α-Ctx contains five disulfide bridges the correct formation of which in mutant should be proved. This may require either determination of spatial structure (e.g., by X-ray or NMR) or combined used of selective disulfide modification and peptide mass fingerprinting. Moreover, the cleavage of some chimeric proteins often used to obtain the protein of interest or direct expression of the protein may add extra amino acid residues at
N-terminus, which may influence the biological activity. We believed that use of natural α-Ctx analogues containing either Arg36 or other residue at this position may be the easiest way to test our hypothesis. If natural α-Ctx analogue with Arg36 is not as active as α-Ctx itself, then this arginine residue is not the only determinant of high α-Ctx affinity to GABA
AR. In this respect, our attention was attracted to the cobra
N. melanoleuca venom in which the presence of two highly homologous long chain neurotoxins 1 and 2 was shown [
23,
24]: neurotoxin 1 contained valine and neurotoxin 2 contained arginine residue at the position corresponding to Arg36 in α-Ctx. They seemed a good pair to clarify the role the respective residue.
In the present work from N. melanoleuca venom we isolated three α-neurotoxins (two with arginine and one with valine at the position corresponding to Arg36 in α-Ctx) and one muscarinic toxin-like protein. Only one neurotoxin (designated here as TX-NM4) was identical to the earlier described N. melanoleuca neurotoxin 2, another (TX-NM3-1) was its analogue with 5 substitutions, while instead of neurotoxin 1 we isolated its analogue with 3 substitutions. Analyzing binding of the isolated toxins to GABAARs and inhibition of the ion currents elicited by GABA, we did not find any evidence for the role of Arg36. However, molecular modeling showed that α-Ctx loop III had contact with α1 subunit of GABAAR and this contact might contribute to the efficient binding of the toxin to the receptor. Thus, our work expands the range of TFTs capable of inhibiting the GABAAR subtypes. In addition, we found that one N. melanoleuca toxin (Tx-NM2) showed unequal affinities for the two ligand-binding sites in the Torpedo californica nAChR and thus widened the range of naturally occurring TFTs that differently interact with the two binding sites in muscle-type nAChRs. Keeping in mind the future modeling and structural studies of the interaction of N. melanoleuca toxins with nAChRs and GABAARs, we investigated the interaction of these toxins with AChBPs from A. californica and L. stagnalis as plausible models. Tx-NM2 was found to be the most potent in binding with both AChPBs.
3. Discussion
Earlier it was shown that several snake venom toxins blocked GABA
AR [
13,
14,
15]. We have found that the snake venom toxins manifested a different efficiency of the interaction with the receptor, α-Ctx being the most active [
15]. As mentioned in the introduction, we suggested that the Arg36 present in α-Ctx (Arg39 in the alignment given in
Figure 2) might determine the highest activity of this toxin against GABA
AR. To check if this is true, we decided to compare the activity of toxins which differ at this position and for this purpose isolated toxins from
N. melanoleuca cobra venom. Two long type α-neurotoxins were isolated earlier from this venom: long neurotoxin 1 (P01383) [
24] containing valine residue at the position 38 (
Figure 2), and long neurotoxin 2 (P01388) [
23] with Arg38 in the sequence (
Figure 2). The only information about biological activity available for these toxins was LD
50 of 1.5 mg/kg for neurotoxin 1 by intraperitoneal injection [
24]. We wanted to get these particular toxins, but in our work we isolated 4 toxins with molecular masses in the range of 7–8 kDa, characteristic for long type α-neurotoxins (
Table 1). Their analysis by high resolution mass spectrometry showed that one of the isolated toxins, designated by us as Tx-NM4, was identical to long neurotoxin 2, while toxin Tx-NM3-1 was long neurotoxin 2 analogue with substitutions in five positions (
Figure 2). Tx-NM2 represented an analogue of
N. melanoleuca long neurotoxin 1 (P01383) and Tx-NM3-2 was homologous to muscarinic toxin-like protein 1 (MTLP-1, P82462) from
N. kaouthia cobra venom. The differences between the published amino acid sequences and those determined in the present work may be explained by different geographical origin of snakes from which the venoms were obtained.
The biological activity of the isolated toxins was assessed against several molecular targets. In the studies on GABA
AR, competition experiments with fluorescently labeled α-Ctx and electrophysiological assays were carried out using several combinations of receptor subunits. Both methods gave the consistent results. Toxin Tx-NM3-1 was the most active on all GABA
AR subtypes studied and manifested activity similar to that of α-Ctx. However, at α1β3γ2 receptor subtype Tx-NM3-1 was less active than α-Ctx displaying IC
50 of 680 nM in contrast to 236 nM for α-Ctx [
15]. Tx-NM2 was slightly less active than Tx-NM3-1 showing IC
50 of 1.25 µM at α1β3γ2 GABA
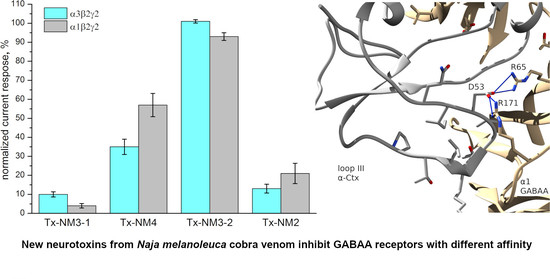
AR. Tx-NM4 was a weaker antagonist than Tx-NM2 and Tx-NM3-1 at all subunit combinations, while Tx-NM3-2 was practically inactive. At the inhibition of α1β3γ2 GABA
AR, the difference in affinity between the most active Tx-NM3-1 and the least active Tx-NM4 was more than one order of magnitude. Tx-NM4 at 10 µM more potently inhibited α1β2γ2 than α3β2γ2 GABA
AR. Thus, the earlier suggestion about essential role of Arg36 (Arg39 in
Figure 2 and
Figure 8) in α-Ctx binding to GABA
AR was not confirmed, as both Tx-NM3-1 and Tx-NM4 contain arginine residue at this position. The analysis of toxin amino acid sequences revealed the identity of loop III sequence in α-Ctx and Tx-NM3-1. Molecular modeling of α-Ctx-GABA
AR complex revealed the contacts between loop III of α-Ctx and α1 subunit of GABA
AR. It was found that Asp56 of α-Ctx loop III forms salt bridges with Arg65 and Arg171 of the α1 subunit, and Thr53 forms a hydrogen bond with Val177 backbone of the α1 subunit (
Figure 9b). The aspartic acid and threonine residues are present at these positions in Tx-NM3-1 as well. The interaction of these residues with α1 subunit of GABA
AR may contribute to the stronger binding of α-Ctx and Tx-NM3-1 to the receptor.
Tx-NM3-2 is the muscarinic toxin-like protein and its amino acid sequence differs greatly from those of the long type neurotoxins to which other studied toxins belong. The muscarinic toxin-like protein was tested on GABA
AR for the first time and it showed some activity, albeit very weak, against α3β2γ2 GABA
AR (
Figure 4). This is the first indication of the activity of muscarinic toxin-like protein against GABA
AR.
The activity of all isolated toxins was studied in competition experiments for binding to
Torpedo and α7 nAChRs as well as to two AChBPs. The neurotoxins tested showed a relatively high affinity to both receptor subtypes. It was found that at
Torpedo nAChR, Tx-NM2 distinguished two binding sites, the affinity differing by about an order of magnitude (
Table 3). Earlier, it was shown that the two binding sites in
Torpedo and muscle types nAChRs were distinguished by αδ-bungarotoxins from the krait
B. candidus venom [
12], the difference being 17-fold for αδ-bungarotoxin-1. Comparison of the amino acid sequences of
N. melanoleuca toxins with those of bungarotoxins and α-Ctx showed that the sequence of Tx-NM2 has a higher identity to that of αδ-bungarotoxin-1 than to α-Btx and α-Ctx (69% versus 61 and 55%, respectively,
Figure 8). At the same time Tx-NM3-1 has 81% identical residues with α-Ctx and only 50% with αδ-bungarotoxin-1. Moreover, the sequence characteristics that were supposed to be responsible for difference in activity between α-Btx and αδ-bungarotoxin-1, i.e., the shortened loop I, the change of Phe residue in position 32 to Trp as well as Arg25 to Thr, are present in Tx-NM2. In addition, only Tx-NM2 and αδ-bungarotoxin-1 contain Tyr residue in position 4 and a positively charged residue in position 5. This similarity in structural features and activity between Tx-NM2 and αδ-bungarotoxin-1 supports the earlier ideas [
12] on the capacity of long chain neurotoxin to distinguish two binding sites in the muscle-type nAChRs. It was found that Tx-NM3-1 was the most active against α7 nAChR (IC
50 4.84 nM), followed by Tx-NM2 and Tx-NM4 with IC
50 of 13.02 and 26.89 nM, respectively. Although the affinities of these toxins for nAChR are much higher than for GABA
AR, their rank coincides with that observed for GABA
AR suggesting that both receptors might have similar structural elements involved in toxin binding to them.
AChBPs are considered as models of the extracellular domains of Cys-loop receptors, in particular of nAChRs. Interestingly, AChBPs has only 20–24% sequence identity with nAChRs, however their pharmacological properties are similar to those of nAChRs. Studies of the binding of N. melanoleuca toxins to AChBPs from L. stagnalis and A. californica showed that toxin Tx-NM2 possessed the highest affinity to both proteins, while toxins Tx-NM3-1 and Tx-NM4 were several orders of magnitude less active. On A. californica AChBP, Tx-NM3-1 was the least active, which is very different from its activity against nAChR and GABAAR, where it was most active. This once again highlights the differences between AChBPs and the extracellular domains of nAChR and the GABAAR.
Considering the above data for nAChRs and AChBPs, one can conclude that for these targets, N. melanoleuca toxins manifest affinities similar to those of α-Btx and α-Ctx, and Tx-NM2 on AChBP from L. stagnalis showed the affinity higher than these two neurotoxins.
To obtain some information about possible molecular mechanisms determining the specificity of TFT interaction with GABAAR, we performed molecular modeling of the complexes formed by N. melanoleuca toxins, α-Bgt and α-Ctx with GABAAR. The modeling showed that the orientation of toxin molecules at GABAAR and nAChRs were different and toxin molecules in complexes with GABAAR tilted towards the complementary subunit. This tilt can lead to close contact of toxin loop III with the α1 subunit of the receptor. More detailed consideration revealed the amino acid residues which can form salt bridges (Asp56 in α-Ctx) and hydrogen bond (Thr53 in α-Ctx) with α1 subunit. These amino acid residues are present in the amino acid sequence of Tx-NM3-1, interacting with GABAAR with affinity similar to that of α-Ctx, but are replaced by other residues in less active toxins. Such substitutions may lead to a significant decrease in the efficiency of toxin interactions with GABAAR.
4. Conclusions
In our previous paper [
15] it was found that among several snake toxins studied only α-Ctx manifested high activity against GABA
AR and we put forward the hypothesis about essential role of Arg36 as the determinant of high affinity to GABA
AR. To check this hypothesis, in this work we additionally studied several snake toxins for their ability to interact with GABA
AR. For this purpose, four toxins were isolated from African cobra
N. melanoleuca venom and their amino acid sequences were established by mass spectrometry. The amino acid sequence of one toxin was identical to that of previously known
N. melanoleuca long neurotoxin 2. The second toxin sequenced differed from that of neurotoxin 2 in five positions. The third one was homologous to
N. melanoleuca long neurotoxin 1; its sequence differed from that of neurotoxin 1 in three positions. One more toxin was homologous to muscarinic toxin-like protein from
N. kaouthia venom and this is the first muscarinic toxin-like protein isolated from African cobra venom. The interaction of
N. melanoleuca toxins with
Torpedo and α7 nAChRs as well as with AChBPs and several subtypes of GABA
ARs was studied. One of isolated toxins, being the most active on AChBPs, manifested different affinity to two binding sites on
Torpedo nAChR. Together with earlier data [
12] this may indicate that there is a group of long type α-neurotoxins capable to bind with different affinity to two binding sites in the muscle type nAChR. All
N. melanoleuca toxins interacted with the GABA
AR much weaker than with the nAChR: one neurotoxin was almost as active as α-Ctx, while others manifested lower activity. The earlier hypothesis about essential role of Arg36 as the sole determinant of high toxin affinity to GABA
AR was not confirmed, but the results of molecular modeling suggest that the loop III may contribute to the efficient interaction of some long-chain neurotoxins with GABA
AR. Experimental proof of the modeling data will be the task of our future work.
5. Materials and Methods
5.1. Materials
All salts obtained from local suppliers were of analytical grade or higher. Venom of cobra N. melanoleuca was from Latoxan (Valence, France). Acetonitrile was purchased from Catrosa Reaktiv LLC (Moscow, Russia), and trifluoroacetic acid from Merck KGaA (Darmstadt, Germany). GH4C1 cells transfected with hα7 nAChR cDNA were a gift of the Eli-Lilly Co. (London, UK). Muscle-type nAChR-enriched membranes from the electric organs of Torpedo californica were kindly provided by Prof. F. Hucho (Free University of Berlin, Germany). Acetylcholine binding proteins from L. stagnalis and A. californica were from Prof. A.B. Smit (Faculty of Earth and Life Sciences, Vrije Universiteit, Amsterdam).
5.2. Isolation of Neurotoxins
A 600 mg sample of dried
N. melanoleuca venom was dissolved in 0.1 M ammonium acetate buffer, pH 6.2, and applied to a Sephadex G50s column (4.5 × 150 cm) equilibrated in the same buffer. The column was eluted at flow rate 32 mL/min. The fractions obtained were pooled as shown in
Figure 1a. Fraction 6 was further separated on a HEMA BIO 1000CM column (4 × 250 mm) (Tessek, Prague, Czech Republic) in an ammonium acetate gradient from 5 to 500 mM (pH 7.5) in 100 min at flow rate 1.0 mL/min (
Figure 1b). Fractions 2, 3 and 4 were freeze-dried and further purified by reversed phase chromatography on Jupiter C18 column (10 × 250 mm, Phenomenex, Torrance, CA, USA) in in a gradient of acetonitrile 20–35% in 60 min in the presence of 0.1% trifluoroacetic acid, at a flow rate of 2.0 mL/min (
Figure S1). After freeze-drying, the obtained proteins were used for further studies.
5.3. Mass Spectrometry Analysis
For mass spectrometry measurements, the carbamidomethylated toxins were digested with trypsin and chymotrypsin at a 1:50 (
w/
w) ratio overnight at 37 °C. Desalting of peptides was carried out using SDB-RPS StageTips that were prepared as described earlier [
33]. After overnight digestion, peptide solution was acidified by equal volume of 2% (
v/
v) TFA and peptides were loaded on SDB-RPS StageTip by centrifugation at 200× g. StageTip was washed by 50 µL 0.2% (
v/
v) TFA and peptides were eluted by 50 μL 50% (
v/
v) acetonitrile, 5% (
v/
v) ammonia, lyophilized, and stored at −80 °C. Before analyses, peptides were dissolved in 20 µL of 2% (
v/
v) acetonitrile, 0.1% (
v/
v) TFA, and sonicated for 2 min. Samples were loaded to a home-made trap column 20 × 0.1 mm, packed with Inertsil ODS3 3 μm sorbent (GLSciences, Tokyo, Japan ), in the loading buffer (2% ACN, 98% H
2O, 0.1% TFA) at 10 μL/min flow and separated at RT in a home-packed [
34] fused-silica column 300 × 0.1 mm packed with Reprosil PUR C18AQ 1.9 (Dr. Maisch, Ammerbuch-Entringen, Germany) into an emitter prepared with P2000 Laser Puller (Sutter Instrument, Novato, CA, USA). Reverse-phase chromatography was performed with an Ultimate 3000 Nano LC System (Thermo Fisher Scientific, Waltham, MA, USA), which was coupled to a Q Exactive Plus benchtop Orbitrap mass spectrometer (Thermo Fisher Scientific) via a nanoelectrospray source (Thermo Fisher Scientific). Peptide samples were eluted with a linear gradient of 80% ACN, 19.9% H
2O, 0.1% FA (buffer B) in 99.9% H
2O, 0.1% FA (solvent A) from 4 to 36% of solvent B in 60 min at 0.5 μL/min flow, intact toxins were separated by linear gradient from 10 to 60% of solvent B in 18 min at 0.5 μL/min flow.
MS raw files were analyzed by PEAKS Studio 8.5 (Waterloo, ON, Canada) [
35] and peak lists were searched against Serpentes Uniprot-Tremble FASTA (canonical and isoform) database version of May 2018 (144954 entries) with cysteine carbamidomethylation as a fixed modification and methionine oxidation and asparagine and glutamine deamidation as variable modifications. Enzyme specificity in the database search was set to trypsin with semi-specific digest mode. False discovery rate was set to 0.01 for peptide-spectrum matches and was determined by searching a reverse database. Peptide identification was performed with an allowed initial precursor mass deviation up to 10 p.p.m. and an allowed fragment mass deviation 0.05 Da.
5.4. Expression of GABAAR in Xenopus Oocytes
Xenopus laevis oocytes were prepared as described [
36]. The work with oocytes was approved by the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry RAS with the approval number IACUC 251/2018 26.02.18. Plasmid DNAs encoding rat GABA
A receptor subunits in pCI mammalian expression vector (Promega, Madison, WI, USA) were kindly provided by Dr. M. Ernst from the Medical University of Vienna. Next day after harvesting oocytes were injected by means of Nanoject II (Drummond Scientific, Broomall, PA, USA) with 2 ng mixture containing vector DNAs encoding receptor α1 or 3, β2 or 3, and γ2 subunits at 1:1:10 mass ratio. Injected oocytes were incubated at 18 °C for 2–3 days in ND96 solution (5 mM HEPES/NaOH pH 7.4, 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl
2, 2 mM MgCl
2) supplemented with gentamycin at 40 μg/mL.
5.5. Two-Electrode Voltage Clamp Electrophysiology Assay
Oocyte was placed in the flow through chamber combined with a nylon grid holding the bath of ND96 solution. Membrane potential of oocyte was clamped at −60mV by TURBO TEC-03X (npi electronic GmbH, Tamm, Germany). Electrodes were pulled from borosilicate capillary (Warner Instruments, Holliston, MA, USA) and filled with 3M KCl solution. Currents were recorded and digitized by WinWCP (University of Strathclyde, Glasgow, UK) software. After stable amplitude of 10 μM GABA-evoked currents was obtained oocyte was pre-incubated with toxin ND96-based solution for three minutes followed by co-application with 10 μM GABA. After steady baseline potential was achieved usually in a five-minute washout session oocyte was perfused with GABA again for current amplitude stability control purposes and pre-incubated with next sample. All solutions were applied manually with automatic pipette in a volume of 200 μL (Eppendorf, Hamburg, Germany). To confirm γ subunit incorporation, GABA response in the presence of 50 μM Zn2⁺ and 1 μM diazepam was tested under the same conditions. Data for toxins are presented as the amplitude ratio of the currents elicited by 10 μM GABA in the presence of toxin to the average of the currents in control elicited by 10 μM GABA before co-application with toxin (100% control current amplitude) in the same oocyte. Data were collected from at least three oocytes from three different batches. Representative current traces, bar graphs and toxin-dependent dose response curves were plotted by Origin 8.1 (OriginLab, Northampton, MA, USA).
5.6. Mammalian Cell Culture
Mouse neuroblastoma Neuro2a cells (Russian collection of cell cultures, Institute of Cytology, Saint Petersburg, Russia) were routinely cultured in incubator (Sanyo, Osaka, Japan) at 37 °C and 5% CO2 in tissue culture treated T25 flasks (SPL, Pocheon, Korea) containing 5 mL Dulbecco’s modified Eagle’s medium (DMEM, Paneco, Moscow, Russia) supplemented with 10% fetal bovine serum (FBS, PAA Laboratories, Pasching, Austria), 50 units/mL streptomycin and 50 µg/mL penicillin. Cells were splitted 1:10 at confluency twice a week non-enzymatically using Versene solution (Paneco, Moscow, Russia).
5.7. Fluorescent Ligand Competition Assay
Neuro2a cells sub-cultured 1:5 24 h before transfection were growing on clear 96-well plate (Corning, Corning, NY, USA) in a complete DMEM (Paneco, Moscow, Russia). Lipofectamine (Invitrogen, Waltham, MA, USA) mediated transfection was performed with equal amounts (0.14 µg/mL) of pCI plasmid expression vectors encoding rat α1, β3, γ2 GABAAR subunits. Transfected Neuro2a cells were grown at 37 °C in 5% CO2 incubator for 72 h, at the day of experiment medium was substituted for extracellular solution containing (in mM) 140 NaCl, 2 CaCl2, 2.8 KCl, 4 MgCl2, 20 HEPES, 10 glucose at pH 7.4. Cells were pre-incubated with 10 µM of toxins for 15 min at room temperature followed by 20 min of incubation with 50 nM Alexa Fluor 546 α-Ctx conjugate in the final volume of 100 µL. Afterwards cells were washed 3 times with two-fold excess of extracellular solution. To control the level of non-specific fluorescence experiment with 10 µM α-Ctx was run under the same conditions. By means of epifluorescent microscope IX71 (Olympus, Tokyo, Japan) equipped with CCD camera pictures of 3 fields chosen on each plate well in bright-field illumination were taken. Fluorescence was counted using CellX and ImageJ open-source software. Intensity of the fluorescence was normalized on integral intensity of the plate well incubated in presence of 50 nM Alexa Fluor 546 α-Ctx conjugate. Each experimental point is an average of integral intensity independently measured on 6 plate wells from three separate passages ± SEM.
5.8. nAChR Competition Radioligand Assay
The competition binding assays with radio-iodinated α-bungarotoxin (
125I-α-Bgt) were performed as in [
37]. Briefly, suspension of GH
4C
1 cells stably transfected with human α7 nAChR (0.4 nM αBgt binding sites) were incubated in 50 μL binding buffer (20 mM Tris-HCl, pH 8.0, containing 1 mg/mL bovine serum albumin) for 90 min with various amounts of toxins. Thereafter, 0.1–0.2 nM
125I-α-Bgt (500 Ci/mmol) was added, and after an additional 5 min incubation, cell suspensions were applied to GF/C glass filters (Cytiva, Marlborough, MA, USA) pretreated with 0.3% polyethyleneimine. The samples were then washed (3 × 4 mL) with 20 mM cold Tris-HCl buffer, pH 8.0, containing 0.1 mg/mL bovine serum albumin and bound radioactivity was measured with a Wallac 1470 Wizard Gamma Counter (PerkinElmer, Waltham, MA, USA). Nonspecific
125I-
αBgt binding was determined in the presence of 200-fold excess of
α-Ctx. The competition binding assays with AChBPs were performed as described in [
38].
5.9. Molecular Modeling
The molecular models of
N. melanoleuca toxin structures were constructed using free bioinformatic tool Swissmodel (
https://swissmodel.expasy.org/ (accessed on 12 February 2021)). α-Ctx structure (PDB 1YI5) was used as template. The molecular model of α-Ctx-GABA
AR complex was taken from [
15]. Briefly, the model of orthosteric binding site was constructed basing on extracellular domain from X-ray structure of β3 GABA
AR subunit [
39]. Swissmoldel service was used to build the extracellular domain of α1 GABA
AR subunit. RMSD between modelled α1 extracellular domain and recently published cryo-EM structure was calculated by UCSF Chimera Match maker tool and did not exceed 1.2 Å. Homology models were submitted to protein docking “Tox Dock” instrument on the Rosetta server [
26,
27] to generate structures of the toxin-GABA
AR complexes. Flexibility of toxin molecules and receptors were taken into account and model structures were subsequently subjected to short molecular dynamics using GROMACS 5.0 package. Briefly, putative structures of TFT-GABA
AR complexes obtained via “Tox Dock” were energy minimized using steepest descent minimization algorithm to a maximum force <1000 kJ/mol/nm. Short-range electrostatic and Van der Waals cut-offs were set to 1 Å, periodic boundary conditions were constructed with rhombododecaedron simulation box having 1.2 Å from protein image to the closest boundary. After energy minimization two consequent constrained 100 ps molecular dynamics were performed to equilibrate systems in NVT (constant number of particles, volume, and temperature) and NPT (constant number of particles, pressure, and temperature) conditions. Equilibration was followed by 100 ps of unconstrained molecular dynamics [
28]. The resulting structures were visualized and inspected in UCSF Chimera [
29]. To compare TFT positions “Match” and “Match-align” functions of UCSF Chimera were used.