Potential of Near Infrared Spectroscopy as a Rapid Method to Discriminate OTA and Non-OTA-Producing Mould Species in a Dry-Cured Ham Model System
Abstract
:1. Introduction
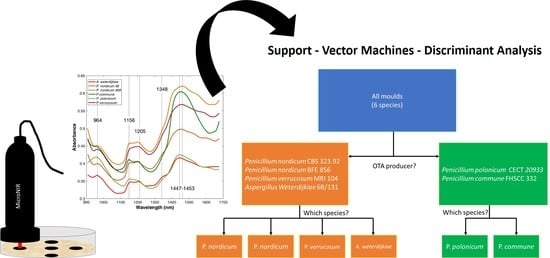
2. Results and Discussion
2.1. Mould Growth Characteristics
2.2. MicroNIR Spectra and Principal Component Analysis
2.3. Classification Models
3. Conclusions
4. Materials and Methods
4.1. Moulds
4.2. Preparation of Moulds Inocula
4.3. Experimental Setting
4.4. MicroNIR Spectra Acquisition
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Magistà, D.; Susca, A.; Ferrara, M.; Logrieco, A.F.; Perrone, G. Penicillium species: Crossroad between quality and safety of cured meat production. Curr. Opin. Food Sci. 2017, 17, 36–40. [Google Scholar] [CrossRef]
- Núñez, F.; Rodríguez, M.M.; Bermúdez, M.E.; Córdoba, J.J.; Asensio, M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 32, 185–197. [Google Scholar] [CrossRef]
- Spotti, E.; Berni, E. Starter cultures: Molds. In Handbook of Fermented Meat and Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 171–176. [Google Scholar]
- Martín, A.; Córdoba, J.J.; Núñez, F.; Benito, M.J.; Asensio, M.A. Contribution of a selected fungal population to proteolysis on dry-cured ham. Int. J. Food Microbiol. 2004, 94, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, T.; Gualla, A.; Morlacchini, M.; Pietri, A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control 2013, 34, 79–83. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Martín, A.; Delgado, J.; Córdoba, J.J. Presence of ochratoxin A on the surface of dry-cured Iberian ham after initial fungal growth in the drying stage. Meat Sci. 2012, 92, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.E.B.; Freire, F.d.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Bignami, M. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar]
- Ministero della Sanità. Direttive in materia di controllo ufficiale sui prodotti alimentari: Valori massimi ammissibili di micotossine nelle derrate alimentari di origine nazionale, comunitaria e Paesi terzi. Gazz. Uff. Della Repubb. Ital. 1999, 135, 52–57. [Google Scholar]
- Cebrián, E.; Rodríguez, M.; Peromingo, B.; Bermúdez, E.; Núñez, F. Efficacy of the combined protective cultures of Penicillium chrysogenum and Debaryomyces hansenii for the control of ochratoxin A hazard in dry-cured ham. Toxins 2019, 11, 710. [Google Scholar] [CrossRef] [Green Version]
- Vipotnik, Z.; Rodríguez, A.; Rodrigues, P. Aspergillus westerdijkiae as a major ochratoxin A risk in dry-cured ham based-media. Int. J. Food Microbiol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Simoncini, N.; Virgili, R.; Spadola, G.; Battilani, P. Autochthonous yeasts as potential biocontrol agents in dry-cured meat products. Food Control 2014, 46, 160–167. [Google Scholar] [CrossRef]
- Sánchez-Montero, L.; Córdoba, J.J.; Peromingo, B.; Álvarez, M.; Núñez, F. Effects of environmental conditions and substrate on growth and ochratoxin A production by Penicillium verrucosum and Penicillium nordicum. Relative risk assessment of OTA in dry-cured meat products. Food Res. Int. 2019, 121, 604–611. [Google Scholar] [CrossRef]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Tao, F.; Yao, H.; Hruska, Z.; Burger, L.W.; Rajasekaran, K.; Bhatnagar, D. Recent development of optical methods in rapid and non-destructive detection of aflatoxin and fungal contamination in agricultural products. Trends Anal. Chem. 2018, 100, 65–81. [Google Scholar] [CrossRef]
- Ferrara, M.; Perrone, G.; Gallo, A.; Epifani, F.; Visconti, A.; Susca, A. Development of loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Penicillium nordicum in dry-cured meat products. Int. J. Food Microbiol. 2015, 202, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Luque, M.I.; Córdoba, J.J.; Rodríguez, A.; Núñez, F.; Andrade, M.J. Development of a PCR protocol to detect ochratoxin A producing moulds in food products. Food Control 2013, 29, 270–278. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Luque, M.I.; Justesen, A.F.; Córdoba, J.J. Quantification of ochratoxin A-producing molds in food products by SYBR Green and TaqMan real-time PCR methods. Int. J. Food Microbiol. 2011, 149, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Goto, T. Near- and mid-infrared spectroscopy as efficient tools for detection of fungal and mycotoxin contamination in agricultural commodities. World Mycotoxin J. 2014, 7, 507–515. [Google Scholar] [CrossRef]
- Yan, H.; Siesler, H.W. Quantitative analysis of a pharmaceutical formulation: Performance comparison of different handheld near-infrared spectrometers. J. Pharm. Biomed. Anal. 2018, 160, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.; Beghi, R.; Giovenzana, V.; Fiorindo, I.; Tugnolo, A.; Guidetti, R. Environmental advantages of visible and near infrared spectroscopy for the prediction of intact olive ripeness. Biosyst. Eng. 2020, 189, 1–10. [Google Scholar] [CrossRef]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Sanchis, V.; Marín, S. Use of hyperspectral imaging as a tool for Fusarium and deoxynivalenol risk management in cereals: A review. Food Control 2020, 108, 106819. [Google Scholar] [CrossRef]
- De Girolamo, A.; von Holst, C.; Cortese, M.; Cervellieri, S.; Pascale, M.; Longobardi, F.; Catucci, L.; Porricelli, A.C.R.; Lippolis, V. Rapid screening of ochratoxin A in wheat by infrared spectroscopy. Food Chem. 2019, 282, 95–100. [Google Scholar] [CrossRef]
- Muszewska, A.; Piłsyk, S.; Perlińska-Lenart, U.; Kruszewska, J.S. Diversity of cell wall related proteins in human pathogenic fungi. J. Fungi 2018, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, M.; Nielsen, P.V.; Martens, H. Near-infrared spectra of Penicillium camemberti strains separated by extended multiplicative signal correction improved prediction of physical and chemical variations. Appl. Spectrosc. 2005, 59, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Braun, S.; Thissen, R.; Dott, W. FT-IR spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J. Microbiol. Methods 2006, 64, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lecellier, A.; Gaydou, V.; Mounier, J.; Hermet, A.; Castrec, L.; Barbier, G.; Ablain, W.; Manfait, M.; Toubas, D.; Sockalingum, G.D. Implementation of an FTIR spectral library of 486 filamentous fungi strains for rapid identification of molds. Food Microbiol. 2015, 45, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Shapaval, V.; Schmitt, J.; Møretrø, T.; Suso, H.P.; Skaar, I.; Åsli, A.W.; Lillehaug, D.; Kohler, A. Characterization of food spoilage fungi by FTIR spectroscopy. J. Appl. Microbiol. 2013, 114, 788–796. [Google Scholar] [CrossRef]
- Kaya-Celiker, H.; Mallikarjunan, P.K.; Kaaya, A. Mid-infrared spectroscopy for discrimination and classification of Aspergillus spp. contamination in peanuts. Food Control 2015, 52, 103–111. [Google Scholar] [CrossRef]
- Kogkaki, E.A.; Sofoulis, M.; Natskoulis, P.; Tarantilis, P.A.; Pappas, C.S.; Panagou, E.Z. Differentiation and identification of grape-associated black aspergilli using Fourier transform infrared (FT-IR) spectroscopic analysis of mycelia. Int. J. Food Microbiol. 2017, 259, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, L.M.; Schwarz, L.J.; Schueuermann, C.; Steel, C.C. Discrimination of Aspergillus spp., Botrytis cinerea, and Penicillium expansum in grape berries by ATR-FTIR spectroscopy. Am. J. Enol. Vitic. 2019, 70, 68–76. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, I. Practical Guide to Interpretive Near-Infrared Spectroscopy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Berardo, N.; Pisacane, V.; Battilani, P.; Scandolara, A.; Pietri, A.; Marocco, A. Rapid detection of kernel rots and mycotoxins in maize by near-infrared reflectance spectroscopy. J. Agric. Food Chem. 2005, 53, 8128–8134. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Fraga, M.E.; Kozakiewicz, Z.; Lima, N. Fourier transform infrared as a powerful technique for the identification and characterization of filamentous fungi and yeasts. Res. Microbiol. 2010, 161, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Ibañez, V.; Soldado, A.; Martínez-Fernández, A.; de la Roza-Delgado, B. Application of near infrared spectroscopy for rapid detection of aflatoxin B1 in maize and barley as analytical quality assessment. Food Chem. 2009, 113, 629–634. [Google Scholar] [CrossRef]
- Serranti, S.; Cesare, D.; Bonifazi, G. The development of a hyperspectral imaging method for the detection of Fusarium-damaged, yellow berry and vitreous Italian durum wheat kernels. Biosyst. Eng. 2013, 115, 20–30. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Kim, M.S.; Dong, Y. Fusarium damage assessment in wheat kernels by Vis/NIR hyperspectral imaging. Sens. Instrum. Food Qual. Saf. 2011, 5, 63–71. [Google Scholar] [CrossRef]
- Williams, P.; Manley, M.; Fox, G.; Geladi, P. Indirect detection of Fusarium verticillioides in maize [Zea mays L kernels by near infrared hyperspectral imaging. J. Near Infrared Spectrosc. 2010, 18, 49–58. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Rodriguez, I.T.; Rausch, S.R.; Graybosch, R.A. Estimating percentages of fusarium-damaged kernels in hard wheat by near-infrared hyperspectral imaging. J. Cereal Sci. 2019, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Pearson, T.C.; Wicklow, D.T.; Pasikatan, M.C. Reduction of aflatoxin and fumonisin contamination in yellow corn by high-speed dual-wavelength sorting. Cereal Chem. 2004, 81, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Da Conceição, R.R.P.; Simeone, M.L.F.; Queiroz, V.A.V.; de Medeiros, E.P.; de Araújo, J.B.; Coutinho, W.M.; da Silva, D.D.; de Araújo Miguel, R.; de Paula Lana, U.G.; de Resende Stoianoff, M.A. Application of near-infrared hyperspectral (NIR) images combined with multivariate image analysis in the differentiation of two mycotoxicogenic Fusarium species associated with maize. Food Chem. 2021, 344, 128615. [Google Scholar] [CrossRef] [PubMed]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]




| Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|
| OTA | NON-OTA | OTA | NON-OTA | ||
| n samples | 63 | 61 | 63 | 61 | |
| Calibration | Class-based | 98 | 100 | 100 | 98 |
| Average-based | 99 | 99 | |||
| n samples | 63 | 61 | 63 | 61 | |
| Cross-validation | Class-based | 95 | 97 | 0.97 | 0.95 |
| Average-based | 96 | 96 | |||
| n samples | 40 | 37 | 40 | 37 | |
| Prediction | Class-based | 76 | 95 | 95 | 76 |
| Average-based | 85 | 86 | |||
| Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|
| P. polonicum | P. commune | P. polonicum | P. commune | ||
| n samples | 30 | 31 | 30 | 31 | |
| Calibration | Class-based | 97 | 84 | 84 | 97 |
| Average-based | 90 | 90 | |||
| n samples | 30 | 31 | 30 | 31 | |
| Cross-validation | Class-based | 100 | 84 | 84 | 100 |
| Average-based | 93 | 93 | |||
| n samples | 18 | 19 | 18 | 19 | |
| Prediction | Class-based | 89 | 100 | 100 | 90 |
| Average-based | 95 | 95 | |||
| Sensitivity (%) | Specificity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P. nordicum 92 | P. nordicum 856 | P. verrucosum | A. weterdijkiae | P. nordicum 92 | P. nordicum 856 | P. verrucosum | A. weterdijkiae | ||
| n samples | 25 | 23 | 20 | 20 | 25 | 23 | 20 | 20 | |
| Calibration | Class-based | 60 | 91 | 70 | 95 | 92 | 91 | 94 | 94 |
| Average-based | 78 | 93 | |||||||
| n samples | 25 | 23 | 20 | 20 | 25 | 23 | 20 | 20 | |
| Cross-validation | Class-based | 60 | 91 | 70 | 90 | 92 | 91 | 91 | 96 |
| Average-based | 77 | 92 | |||||||
| n samples | 10 | 13 | 18 | 17 | 10 | 13 | 18 | 17 | |
| Prediction | Class-based | 50 | 85 | 56 | 82 | 92 | 87 | 95 | 85 |
| Average-based | 69 | 90 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebrián, E.; Núñez, F.; Rodríguez, M.; Grassi, S.; González-Mohino, A. Potential of Near Infrared Spectroscopy as a Rapid Method to Discriminate OTA and Non-OTA-Producing Mould Species in a Dry-Cured Ham Model System. Toxins 2021, 13, 620. https://doi.org/10.3390/toxins13090620
Cebrián E, Núñez F, Rodríguez M, Grassi S, González-Mohino A. Potential of Near Infrared Spectroscopy as a Rapid Method to Discriminate OTA and Non-OTA-Producing Mould Species in a Dry-Cured Ham Model System. Toxins. 2021; 13(9):620. https://doi.org/10.3390/toxins13090620
Chicago/Turabian StyleCebrián, Eva, Félix Núñez, Mar Rodríguez, Silvia Grassi, and Alberto González-Mohino. 2021. "Potential of Near Infrared Spectroscopy as a Rapid Method to Discriminate OTA and Non-OTA-Producing Mould Species in a Dry-Cured Ham Model System" Toxins 13, no. 9: 620. https://doi.org/10.3390/toxins13090620
APA StyleCebrián, E., Núñez, F., Rodríguez, M., Grassi, S., & González-Mohino, A. (2021). Potential of Near Infrared Spectroscopy as a Rapid Method to Discriminate OTA and Non-OTA-Producing Mould Species in a Dry-Cured Ham Model System. Toxins, 13(9), 620. https://doi.org/10.3390/toxins13090620