Industrial-Scale Cleaning Solutions for the Reduction of Fusarium Toxins in Maize
Abstract
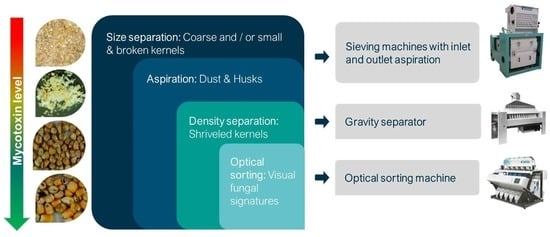
:1. Introduction
2. Results
2.1. First Study
2.2. Second Study
2.3. Mass Balance
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Reagents
5.2. Samples and Cleaning Processes
5.3. Sampling
5.4. Mycotoxins Analysis
5.4.1. Analysis of DON
5.4.2. Analysis of ZEA
5.4.3. Analysis of FB1 and FB2
5.5. Mass Balance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin Production in Fusarium According to Contemporary Species Concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Bulder, A.S.; DiNovi, M.; Kpodo, K.A.; Leblanc, J.-C.; Resnik, S.; Shephard, G.S.; Slob, W.; Walker, R.; Wolterink, G. Deoxynivalenol (addendum). In Safety Evaluation of Certain Contaminants in Food (Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives, JECFA); WHO Food Additives Series No. 63; FAO JECFA Monographs 8; World Health Organization: Geneva, Switzerland, 2011; pp. 317–485. [Google Scholar]
- Eriksen, G.S.; Pennington, J.; Schlatter, J.; Alexander, J.; Thuvander, A. Zearalenone. In Safety Evaluation of Certain Food Additives and Contaminants (Prepared by the Fifty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, JECFA); WHO Food Additives Series No. 44; IPCS—International Programme on Chemical Safety; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Riley, R.T.; Edwards, S.G.; Aidoo, K.; Alexander, J.; Bolger, M.; Boon, P.E.; Cressey, P.; Doerge, D.R.; Edler, L.; Miller, J.D.; et al. Fumonisins (addendum). In Safety Evaluation of Certain Contaminants in Food (Prepared by Eighty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, JECFA); WHO Food Additives Series No. 74; FAO JECFA Monographs 19 bis; World Health Organization: Geneva, Switzerland, 2018; pp. 415–574. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Scientific Opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on the risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [Green Version]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on the risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Press: Lyon, France, 2002; Volume 82, pp. 301–366. [Google Scholar]
- van Egmond, H.P.; Jonker, M.A. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO Food and Nutrition Paper 81; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Commission of the European Communities. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, 255, 14–17. [Google Scholar]
- Commission of the European Communities. Commission Recommendation (2006/576/EC) of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Commission of the European Communities0. Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food. Off. J. Eur. Union 2016, 208, 58–60. [Google Scholar]
- Commission of the European Communities. Commission Recommendation on prevention and reduction of Fusarium toxins in cereals. Off. J. Eur. Union 2006, 234, 35–40. [Google Scholar]
- CODEX ALIMENTARIUS CXC 51-2003 (Amended in 2017) “Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals”. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B51-2003%252FCXC_051e.pdf (accessed on 29 July 2022).
- Reyneri, A.; Bruno, G.; D’Egidio, M.G.; Balconi, C. Guidelines for the Control of Mycotoxins in Maize and Wheat, 2015, MIPAAF, Ministry of Agriculture, Food and Forestry Policies (In Italian). Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/9703 (accessed on 29 July 2022).
- Grenier, B.; Loureiro-Bracarense, A.-P.; Leslie, J.F.; Oswald, I.P. Physical and Chemical Methods for mycotoxin decontamination in maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley and Sons, Inc.: Ames, IA, USA, 2004; pp. 116–129. [Google Scholar]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Colovic, R.; Puvaca, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Duragic, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Odjo, S.; Alakonya, A.E.; Rosales-Nolasco, A.; Molina, A.L.; Munoz, C.; Palacios-Rojas, N. Occurrence and postharvest strategies to help mitigate aflatoxins and fumonisins in maize and their co-exposure to consumers in Mexico and Central America. Food Control 2022, 138, 108968. [Google Scholar] [CrossRef]
- Pinton, P.; Suman, M.; Buck, N.; Dellafiora, L.; De Meester, J.; Stadler, D.; Rito, E. Practical Guidance to Mitigation of Mycotoxins during Food Processing; ILSI Europe Report Series 2019; ILSI Europe: Brussels, Belgium, 2019; Available online: https://ilsi.eu/publication/practical-guidance-to-mitigation-of-mycotoxins-during-food-processing (accessed on 29 July 2022)ISBN 9789078637455.
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Hoffmans, Y.; Schaarschmidt, S.; Fauhl-Hassek, C.; van der Fels-Klerx, H.J. Factors during Production of Cereal-Derived Feed That Influence Mycotoxin Contents. Toxins 2022, 14, 301. [Google Scholar] [CrossRef]
- Shi, H.; Stroshine, R.; Ileleji, K. Aflatoxin reduction in corn by cleaning and sorting. In Proceedings of the American Society of Agricultural and Biological Engineers, Annual International Meeting 2014, Montreal, QC, Canada, 13–16 July 2014; Volume 1, pp. 311–321. [Google Scholar]
- Shi, H.; Stroshine, R.L.; Ileleji, K. Differences in kernel shape, size, and density between healthy kernels and mold discolored kernels and their relationship to reduction in aflatoxin levels in a sample of shelled corn. Appl. Eng. Agric. 2017, 33, 421–431. [Google Scholar] [CrossRef]
- Ngure, F.M.; Ngure, C.; Achieng, G.; Munga, F.; Moran, Z.; Stafstrom, W.; Nelson, R.J. Mycotoxins contamination of market maize and the potential of density sorting in reducing exposure in unregulated food systems in Kenya. World Mycotoxin. J. 2020, 14, 165–178. [Google Scholar] [CrossRef]
- Sydenham, E.W.; Van der Westhuizen, L.; Stockenström, S.; Shephard, G.S.; Thiel, P.G. Fumonisin-contaminated maize: Physical treatment for the partial decontamination of bulk shipments. Food Addit. Contam. 1994, 11, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Stafstroma, W.; Priest, P.; John Fuchs, J.; Windhamd, G.L.; Williams, P.W.; Nelson, R.J. Low-cost grain sorting technologies to reduce mycotoxin contamination in maize and groundnut. Food Control 2020, 118, 107363. [Google Scholar] [CrossRef] [PubMed]
- Carmack, W.J.; Clark, A.J.; Dong, Y.; van Sanford, D.A. Mass Selection for Reduced Deoxynivalenol Concentration Using an Optical Sorter in SRW Wheat. Agronomy 2019, 9, 816. [Google Scholar] [CrossRef] [Green Version]
- Nagy, E.; Korzenszky, P.; Sembery, P. The role of color sorting machine in reducing food safety risks. Potravinarstvo 2016, 10, 354–358. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Falade, T.D.O.; Mutuma, M.; Mutiga, S.K.; Harvey, J.J.W.; Fox, G.; Pearson, T.C.; Muthomi, J.W.; Nelson, R.J. Multi-spectral kernel sorting to reduce aflatoxins and fumonisins in Kenyan maize. Food Control 2017, 78, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef] [Green Version]
- Visconti, A.; Haidukowski, M.; Pascale, M.; Silvestri, M. Reduction of deoxynivalenol during durum wheat processing and spaghetti cooking. Toxicol. Lett. 2004, 153, 181–189. [Google Scholar] [CrossRef]
- Pascale, M.; Haidukowski, M.; Lattanzio, V.M.T.; Silvestri, M.; Ranieri, R.; Visconti, A. Distribution of T-2 and HT-2 Toxins in Milling Fractions of Durum Wheat. J. Food Prot. 2011, 74, 1700–1707. [Google Scholar] [CrossRef] [Green Version]
- Tibola, C.S.; Fernandes, J.M.C.; Guarienti, E.M. Effect of cleaning, sorting and milling processes in wheat mycotoxin content. Food Control 2016, 60, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Brodal, G.; Aamot, H.U.; Almvik, M.; Hofgaard, I.S. Removal of Small Kernels Reduces the Content of Fusarium Mycotoxins in Oat Grain. Toxins 2020, 12, 346. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during the primary food processing of maize. Food Control 2021, 121, 107651. [Google Scholar] [CrossRef]
- Fandohan, P.; Zoumenou, D.; Hounhouigan, D.J.; Marasas, W.F.O.; Wingfield, M.J.; Hell, K. Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. Int. J. Food Microbiol. 2005, 98, 249–259. [Google Scholar] [CrossRef]
- Matumba, L.; Van Poucke, C.; Ediage, E.N.; Jacobs, B.; De Saeger, S. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize. Food Addit. Contam. Part A 2015, 32, 960–969. [Google Scholar] [CrossRef]
- van der Westhuizen, L.; Shephard, G.S.; Rheeder, J.P.; Burger, H.M.; Gelderblom, W.C.A.; Wild, C.P.; Gong, Y.Y. Optimising sorting and washing of home-grown maize to reduce fumonisin contamination under laboratory-controlled conditions. Food Control 2011, 22, 396–400. [Google Scholar] [CrossRef]
- Pacin, A.M.; Resnik, S.L. Reduction of mycotoxin contamination by segregation with sieves prior to maize milling. In Novel Technologies in Food Science: Their Impact on Products, Consumer Trends and the Environment; McElhatton, A., do Amaral Sobral, P.J., Eds.; Springer: New York, NY, USA, 2012; pp. 219–234. [Google Scholar]
- Yoder, A.; Tokach, M.D.; DeRouchey, J.M.; Paulk, C.B. Cleaning reduces mycotoxin contamination in corn. Kans. Agric. Exp. Stn. Res. Rep. 2017, 3, 50. [Google Scholar] [CrossRef] [Green Version]
- Yoder, A.D.; Stark, C.R.; DeRouchey, J.M.; Tokach, M.D.; Jones, C.K. Mechanically cleaning corn reduces fumonisin concentration. J. Anim. Sci. 2018, 96 (Suppl. 2), 87–88. [Google Scholar] [CrossRef]
- Pearson, T.C.; Wicklow, D.T.; Pasikatan, M.C. Reduction of aflatoxin and fumonisin contamination in yellow corn by high-speed dual-wavelength sorting. Cereal Chem. 2004, 81, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Pearson, T.C.; Wicklow, D.T.; Brabec, D.L. Characteristics and sorting of white food corn contaminated with mycotoxins. Appl. Eng. Agric. 2010, 26, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Drochner, W. Redistribution of 16 Fusarium toxins during commercial dry milling of maize. Cereal Chem. J. 2008, 85, 557–560. [Google Scholar] [CrossRef]
- Scarpino, V.; Vanara, F.; Reyneri, A.; Blandino, M. Fate of moniliformin during different large-scale maize dry-milling processes. LWT-Food Sci. Technol. 2020, 123, 109098. [Google Scholar] [CrossRef]
- Pietri, A.; Zanetti, M.; Bertuzzi, T. Distribution of aflatoxins and fumonisins in dry-milled maize fractions. Food Addit. Contam. Part A 2009, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, M.; Logrieco, A.F.; Graeber, M.; Hirschberger, M.; Reichel, M.; Lippolis, V.; De Girolamo, A.; Lattanzio, V.M.T.; Slettengren, K. Aflatoxin Reduction in Maize by Industrial-Scale Cleaning Solutions. Toxins 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT-Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Delwiche, S.R. High-speed optical sorting of soft wheat for reduction of deoxynivalenol. Plant Dis. 2005, 89, 1214–1219. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Ishibashi, J.; Miyamoto, T.; Tateishi, Y.; Ito, T.; Hara, M.; Kawano, M.; Nakajima, T.; Yoshida, M.; Kawamura, T.; et al. Reduction of Wheat DON and NIV Concentrations with Optical Sorters. Trans. ASAE Am. Soc. Agric. Eng. 2009, 52, 859–866. [Google Scholar] [CrossRef]
- Generotti, S.; Cirlini, M.; Dall’Asta, C.; Suman, M. Influence of the industrial process from caryopsis to cornmeal semolina on levels of fumonisins and their masked forms. Food Control 2015, 48, 170–174. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 401/2006, of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Ciasca, B.; De Saeger, S.; De Boevre, M.; Reichel, M.; Pascale, M.; Logrieco, A.F.; Lattanzio, V.M.T. Mycotoxin Analysis of Grain via Dust Sampling: Review, Recent Advances and the Way Forward: The Contribution of the MycoKey Project. Toxins 2022, 14, 381. [Google Scholar] [CrossRef]
- MacDonald, S.J.; Chan, D.; Brereton, P.; Damant, A.; Wood, R. Determination of Deoxynivalenol in Cereals and Cereal Products by Immunoaffinity Column Cleanup with Liquid Chromatography: Interlaboratory Study. J. AOAC Int. 2005, 88, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, S.; Anderson, S.; Brereton, P.; Wood, R.; Damant, A. Determination of zearalenone in barley, maize and wheat flour, polenta, and maize-based baby food by immunoaffinity column cleanup with liquid chromatography: Interlaboratory study. J. AOAC Int. 2005, 88, 1733–1740. [Google Scholar] [CrossRef] [Green Version]
- Visconti, A.; Solfrizzo, M.; De Girolamo, A. Determination of Fumonisins B1 and B2 in Corn and Corn Flakes by Liquid Chromatography with Immunoaffinity Column Cleanup: Collaborative Study. J. AOAC Int. 2001, 84, 1828–1837. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo, A.; Pascale, M.; Visconti, A. Comparison of methods and optimisation of the analysis of fumonisins B1 and B2 in masa flour, an alkaline cooked corn product. Food Addit. Contam. Part A 2011, 28, 667–675. [Google Scholar] [CrossRef]



| Maize-Cleaning Fraction | Study 1 | Study 2 | |||
|---|---|---|---|---|---|
| Sampled Fraction | Yield (%) Batches A–C | Sampled Fraction | Yield (%) Batches A1, A2 | Yield (%) Batches B1, B2 | |
| Unprocessed raw maize a | 1 | 100 | 1 | 100 | 100 |
| Rejected fraction from separator b | - | 2 | 3.0 | 2.0 | |
| Rejected fraction from separator c | - | 3 | 0.9 | 0.9 | |
| Rejected fraction from aspirator d | - | 4 | 0.5 | 0.5 | |
| Rejected fraction from concentrator e | - | 5 | 1.5 | 0.5 | |
| Rejected fraction from optical sorter f | 2 | 5.0 | 6 | 0.5 | 0.1 |
| Cleaned maize | 3 | 95.0 | 7 | 93.6 | 96.0 |
| Batch | Sampled Fraction 1 | DON (µg/kg) | DON Reduction (%) | ZEA (µg/kg) | ZEA Reduction (%) | FBs 2 (µg/kg) | FBs 2 Reduction (%) |
|---|---|---|---|---|---|---|---|
| A | 1 | 11,130 | 63 | 2690 | 78 | 5680 | 27 |
| 2 | 108,540 | 40,310 | 22,850 | ||||
| 3 | 4100 | 580 | 4150 | ||||
| B | 1 | 17,400 | 67 | 4460 | 87 | 6540 | 28 |
| 2 | 168,250 | 18,700 | 22,320 | ||||
| 3 | 5790 | 590 | 4690 | ||||
| C | 1 | 3200 | 44 | 660 | 67 | 2520 | 27 |
| 2 | 27,620 | 10,060 | 7860 | ||||
| 3 | 1780 | 220 | 1830 |
| Batch | Sampled Fraction 1 | DON (µg/kg) | DON Reduction (%) | ZEA (µg/kg) | ZEA Reduction (%) | FBs 2 (µg/kg) | FBs 2 Reduction (%) |
|---|---|---|---|---|---|---|---|
| A1 | 1 | 250 | 36 | 50 | 80 | 1705 | 54 |
| 2 | 940 | 160 | 7640 | ||||
| 3 | 1260 | 265 | 14,180 | ||||
| 4 | 1350 | 270 | 8100 | ||||
| 5 | 2600 | 540 | 31,760 | ||||
| 6 | 5550 | 90 | 3640 | ||||
| 7 | 160 | 10 | 780 | ||||
| A2 | 1 | 220 | 52 | 40 | 75 | 1765 | 34 |
| 2 | 680 | 170 | 6830 | ||||
| 3 | 1490 | 230 | 19,720 | ||||
| 4 | 1300 | 130 | 17,305 | ||||
| 5 | 1560 | 350 | 28,890 | ||||
| 6 | 2550 | 490 | 5620 | ||||
| 7 | 105 | 10 | 1160 | ||||
| B1 | 1 | 350 | 43 | 55 | 82 | 1740 | 67 |
| 2 | 1080 | 115 | 4870 | ||||
| 3 | 4240 | 550 | 48,250 | ||||
| 4 | 10,680 | 1405 | 35,575 | ||||
| 5 | 5760 | 760 | 52,530 | ||||
| 6 | 12,280 | 750 | 8240 | ||||
| 7 | 200 | 10 | 580 | ||||
| B2 | 1 | 330 | 48 | 50 | 80 | 1735 | 50 |
| 2 | 1030 | 55 | 4740 | ||||
| 3 | 4970 | 560 | 44,980 | ||||
| 4 | 9900 | 180 | 40,670 | ||||
| 5 | 5820 | 250 | 39,990 | ||||
| 6 | 11,230 | 745 | 7400 | ||||
| 7 | 170 | 10 | 860 |
| Study | Batch | DON (%) | ZEA (%) | FBs 1 (%) |
|---|---|---|---|---|
| 1 | A | 84 | 95 | 90 |
| B | 80 | 87 | 85 | |
| C | 96 | 108 | 85 | |
| 2 | A1 | 105 | 53 | 95 |
| A2 | 79 | 62 | 114 | |
| B1 | 99 | 43 | 88 | |
| B2 | 96 | 37 | 100 |
| Study | Sampled Fraction | Number of Incremental Samples | Aggregate Sample Weight (kg) |
|---|---|---|---|
| 1 1 | 1, 3 | 100 | 10–14 |
| 2 | 10 | 1–2 | |
| 2 2 | 1, 7 | 60 | 6–10 |
| 2 | 10 | 1–2 | |
| 3, 4, 5, 6 | 5 | 1–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascale, M.; Logrieco, A.F.; Lippolis, V.; De Girolamo, A.; Cervellieri, S.; Lattanzio, V.M.T.; Ciasca, B.; Vega, A.; Reichel, M.; Graeber, M.; et al. Industrial-Scale Cleaning Solutions for the Reduction of Fusarium Toxins in Maize. Toxins 2022, 14, 728. https://doi.org/10.3390/toxins14110728
Pascale M, Logrieco AF, Lippolis V, De Girolamo A, Cervellieri S, Lattanzio VMT, Ciasca B, Vega A, Reichel M, Graeber M, et al. Industrial-Scale Cleaning Solutions for the Reduction of Fusarium Toxins in Maize. Toxins. 2022; 14(11):728. https://doi.org/10.3390/toxins14110728
Chicago/Turabian StylePascale, Michelangelo, Antonio F. Logrieco, Vincenzo Lippolis, Annalisa De Girolamo, Salvatore Cervellieri, Veronica M. T. Lattanzio, Biancamaria Ciasca, Anna Vega, Mareike Reichel, Matthias Graeber, and et al. 2022. "Industrial-Scale Cleaning Solutions for the Reduction of Fusarium Toxins in Maize" Toxins 14, no. 11: 728. https://doi.org/10.3390/toxins14110728
APA StylePascale, M., Logrieco, A. F., Lippolis, V., De Girolamo, A., Cervellieri, S., Lattanzio, V. M. T., Ciasca, B., Vega, A., Reichel, M., Graeber, M., & Slettengren, K. (2022). Industrial-Scale Cleaning Solutions for the Reduction of Fusarium Toxins in Maize. Toxins, 14(11), 728. https://doi.org/10.3390/toxins14110728