Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications
Abstract
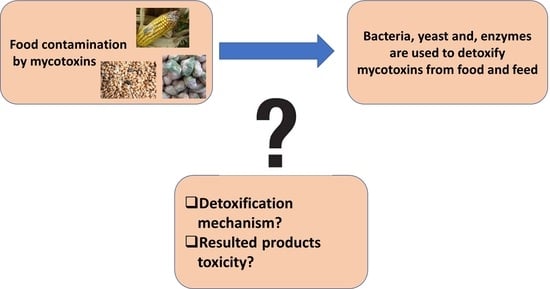
:1. Introduction
2. Major Mycotoxin Overview
3. Microorganism Degradation
3.1. Toxin Detoxification by Bacteria
3.2. Mycotoxin Detoxification by Yeast
3.3. Toxin Detoxification by Enzymes
4. Detoxification Mechanism
4.1. Biodegradation Mechanism
4.2. Decontamination by Removal Mechanism
4.3. Degradation Compound Toxicity
5. Functional Enzymes Extraction from Bacteria
6. Application and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Ünüsan, N. Systematic review of mycotoxins in food and feeds in Turkey. Food Control 2019, 97, 1–14. [Google Scholar] [CrossRef]
- Hoyos Ossa, D.E.; Hincapié, D.A.; Peñuela, G.A. Determination of aflatoxin M1 in ice cream samples using immunoaffinity columns and ultra-high performance liquid chromatography coupled to tandem mass spectrometry. Food Control 2015, 56, 34–40. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Zalán, Z.; Mörtl, M.; Juracsek, J.; Szendrő, I.; Székács, A.; Adányi, N. Optical waveguide lightmode spectroscopy technique-based immunosensor development for aflatoxin B1 determination in spice paprika samples. Food Chem. 2016, 211, 972–977. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hu, X.; Zhang, Q.; Li, P. Determination for multiple mycotoxins in agricultural products using HPLC-MS/MS via a multiple antibody immunoaffinity column. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1021, 145–152. [Google Scholar] [CrossRef]
- Mwakinyali, S.E.; Ding, X.; Ming, Z.; Tong, W.; Zhang, Q.; Li, P. Recent development of aflatoxin contamination biocontrol in agricultural products. Biol. Control 2019, 128, 31–39. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Lizarraga, E.; González-peñas, E.; López, A.; Cerain, D. Co-occurrence of type-A and type-B trichothecenes in barley from a northern region of Spain. Food Control 2012, 25, 81–88. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J. Analysis of E.U. Rapid Alert System (RASFF) Notifications for Aflatoxins in Exported U.S. Food and Feed Products for 2010–2019. Toxins 2021, 13, 90. [Google Scholar] [CrossRef]
- Chala, A.; Taye, W.; Ayalew, A.; Krska, R.; Sulyok, M.; Logrieco, A. Multimycotoxin analysis of sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Food Control 2014, 45, 29–35. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Sulyok, M.; Warth, B.; Odebode, A.C.; Krska, R. Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control 2012, 27, 338–342. [Google Scholar] [CrossRef]
- Monyo, E.; Njoroge, S.; Coe, R.; Osiru, M.; Madinda, F.; Waliyar, F.; Thakur, R.; Chilunjika, T.; Anitha, S. Occurrence and distribution of aflatoxin contamination in groundnuts (Arachis hypogaea L.) and population density of Aflatoxigenic Aspergilli in Malawi. Crop Prot. 2012, 42, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Hajok, I.; Kowalska, A.; Piekut, A.; Ćwieląg-Drabek, M. A risk assessment of dietary exposure to ochratoxin A for the Polish population. Food Chem. 2019, 284, 264–269. [Google Scholar] [CrossRef]
- Zafar, S.; Ra, M.; Jinap, S.; Rashid, U. Detection of a fl atoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar]
- Del, E.M.; Garda-buffon, J.; Badiale-furlong, E. Deoxynivalenol and nivalenol in commercial wheat grain related to Fusarium head blight epidemics in southern Brazil. Food Chem. 2012, 132, 1087–1091. [Google Scholar]
- Yang, X.; Zhang, Q.; Chen, Z.Y.; Liu, H.; Li, P. Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE 2017, 12, e0178810. [Google Scholar] [CrossRef] [Green Version]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226. [Google Scholar] [CrossRef]
- Marković, M.; Daković, A.; Rottinghaus, G.E.; Kragović, M.; Petković, A.; Krajišnik, D.; Milić, J.; Mercurio, M.; de Gennaro, B. Adsorption of the mycotoxin zearalenone by clinoptilolite and phillipsite zeolites treated with cetylpyridinium surfactant. Colloids Surf. B Biointerfaces 2017, 151, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Cserháti, M.; Kriszt, B.; Krifaton, C.; Szoboszlay, S.; Háhn, J.; Tóth, S.; Nagy, I.; Kukolya, J. Mycotoxin-degradation profile of Rhodococcus strains. Int. J. Food Microbiol. 2013, 166, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Fate of Fusarium mycotoxins during processing of Nigerian traditional infant foods (ogi and soybean powder). Food Res. Int. 2019, 116, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, J.A.; Kayitesi, E.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Food fermentation and mycotoxin detoxification: An African perspective. Food Control 2019, 106, 106731. [Google Scholar] [CrossRef]
- Peng, W.-X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Prettl, Z.; Dési, E.; Lepossa, A.; Kriszt, B.; Kukolya, J.; Nagy, E. Biological degradation of aflatoxin B1by a Rhodococcus pyridinivorans strain in by-product of bioethanol. Anim. Feed Sci. Technol. 2017, 224, 104–114. [Google Scholar] [CrossRef]
- Li, M.; Chen, W.; Zhang, Z.; Zhang, Z.; Peng, B. Fermentative degradation of Patulin by Saccharomyces cerevisiae in aqueous solution. LWT—Food Sci. Technol. 2018, 97, 427–433. [Google Scholar] [CrossRef]
- Harkai, P.; Szabó, I.; Cserháti, M.; Krifaton, C.; Risa, A.; Radó, J.; Balázs, A.; Berta, K.; Kriszt, B. Biodegradation of aflatoxin-B1 and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegrad. 2016, 108, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Siri-Anusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The Occurrence and Co-Occurrence of Regulated, Emerging, and Masked Mycotoxins in Rice Bran and Maize from Southeast Asia. Toxins 2022, 14, 567. [Google Scholar] [CrossRef]
- Xie, J.; Sun, Y.; Zheng, Y.; Wang, C.; Sun, S.; Li, J.; Ding, S.; Xia, X.; Jiang, H. Preparation and application of immunoaffinity column coupled with dcELISA detection for aflatoxins in eight grain foods. Food Control 2017, 73, 445–451. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Y.; Shen, Q.; Liu, R.; Shi, R.; Wang, H.; Yang, Z. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta 2019, 195, 55–61. [Google Scholar] [CrossRef]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; Freire, F.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Xie, J.; Peng, T.; He, J.L.; Shao, Y. Preparation and characterization of an immunoaffinity column for the selective extraction of aflatoxin B1 in 13 kinds of foodstuffs. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 998–999, 50–56. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, L.; Zhu, Y.; Huang, Y.; Hu, X.; Wu, Q.; Nüssler, A.K.; Liu, L.; Yang, W. Current major degradation methods for aflatoxins: A review. Trends Food Sci. Technol. 2018, 80, 155–166. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hajimohammadi, A.; Badiei, K.; Pourjafar, M.; Naserian, A.A.; Razavi, S.A. Effect of dietary supplementation of bentonite and yeast cell wall on serum endotoxin, inflammatory parameters, serum and milk aflatoxin in high-producing dairy cows during the transition period. Comp. Clin. Path. 2020, 29, 433–440. [Google Scholar] [CrossRef]
- WHO/FAO. General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193-1995); World Health Organization: Geneva, Switzerland, 2015; Volume 51, pp. 39–54. [Google Scholar]
- Engin, A.B.; Engin, A. DNA damage checkpoint response to aflatoxin B1. Environ. Toxicol. Pharmacol. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Sanchis, V.; Viñas, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Formation of patulin-glutathione conjugates induced by pulsed light: A tentative strategy for patulin degradation in apple juices. Food Chem. 2020, 315, 126283. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, Q.; Hu, N. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. Toxicol. 2019, 270, 1–9. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate–cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin A production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Sohrabi, H.; Arbabzadeh, O.; Khaaki, P.; Khataee, A.; Majidi, M.R.; Orooji, Y. Patulin and Trichothecene: Characteristics, occurrence, toxic effects and detection capabilities via clinical, analytical and nanostructured electrochemical sensing/biosensing assays in foodstuffs. Crit. Rev. Food Sci. Nutr. 2022, 62, 5540–5568. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Z.; Yuan, Y.; Yue, T. Survey of patulin in apple juice concentrates in Shaanxi (China) and its dietary intake. Food Control 2013, 34, 570–573. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Christou, O.; Tsolakidou, M.D.; Tsaltas, D.; Ioannou, N. Isolation, identification and in vitro screening of grapevine yeasts for the control of black aspergilli on grapes. Biol. Control 2015, 88, 46–53. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Marini, A.; Tosi, L. A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-forti fi ed dessert wines and dried vine fruit in the Mediterranean area. Food Control 2012, 26, 347–356. [Google Scholar] [CrossRef]
- Peromingo, B.; Núñez, F.; Rodríguez, A.; Alía, A.; Andrade, M.J. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018, 268, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Microbiology Impact of some environmental factors on growth and ochratoxin A production by Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2019, 291, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Ponsone, M.L.; Nally, M.C.; Chiotta, M.L.; Combina, M.; Köhl, J.; Chulze, S.N. Evaluation of the effectiveness of potential biocontrol yeasts against black sur rot and ochratoxin A occurring under greenhouse and field grape production conditions. Biol. Control 2016, 103, 78–85. [Google Scholar] [CrossRef]
- Zhang, H.; Apaliya, M.T.; Mahunu, G.K.; Chen, L.; Li, W. Trends in Food Science & Technology Control of ochratoxin A-producing fungi in grape berry by microbial antagonists: A review. Trends Food Sci. Technol. 2016, 51, 88–97. [Google Scholar]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Khoi, C.; Chen, J.; Lin, T.; Chiang, C. Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, W.; Ryu, S.; Kim, J.; Song, G. Ochratoxin A mediates cytotoxicity through the MAPK signaling pathway and alters intracellular homeostasis in bovine mammary epithelial cells. Environ. Pollut. 2019, 246, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Pexara, A.; Solomakos, N.; Govaris, A. Ochratoxin A in Slaughtered Pigs and Pork Products. Toxins 2022, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Abbas, F.; Rhouati, A.; Sun, Y.; Chu, X.; Cui, S.; Sun, B.; Xue, C. Design of a Quencher-Free Fluorescent Aptasensor for Ochratoxin A Detection in Red Wine Based on theGuanine-Quenching Ability. Biosensors 2022, 12, 297. [Google Scholar]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin b1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Liu, X.; Fan, L.; Yin, S.; Chen, H.; Hu, H. Molecular mechanisms of fumonisin B1-induced toxicities and its applications in the mechanism-based interventions. Toxicon 2019, 167, 1–5. [Google Scholar] [CrossRef]
- Arumugam, T.; Pillay, Y.; Ghazi, T.; Nagiah, S.; Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1-induced oxidative stress triggers Nrf2-mediated antioxidant response in human hepatocellular carcinoma (HepG2) cells. Mycotoxin Res. 2019, 35, 99–109. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25 %. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Gao, X.; Mu, P.; Wen, J.; Sun, Y.; Chen, Q.; Deng, Y. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018, 112, 310–319. [Google Scholar] [CrossRef]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; Almeida, F.G.; Minella, E.; Corrêa, B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018, 34, 173–178. [Google Scholar] [CrossRef]
- dos Santos, J.S.; Souza, T.M.; Ono, E.Y.S.; Hashimoto, E.H.; Bassoi, M.C.; de Miranda, M.Z.; Itano, E.N.; Kawamura, O. Natural occurrence of deoxynivalenol in wheat from Paraná State, Brazil and estimated daily intake by wheat products. Food Chem. 2013, 138, 90–95. [Google Scholar] [CrossRef]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [Green Version]
- Bonnighausen, J.; Schauer, N.; Schafer, W.; Bormann, J. Metabolic profiling of wheat rachis node infection by Fusarium graminearum—Decoding deoxynivalenol-dependent susceptibility. New Phytol. 2019, 221, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajecka, M.; Gajecki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Frizzell, C.; Uhlig, S.; Miles, C.O.; Verhaegen, S.; Elliott, C.T.; Eriksen, G.S.; Sørlie, M.; Ropstad, E. Toxicology in Vitro Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol. In Vitro 2015, 29, 575–581. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafi, K.; Walczak, J. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018, 410, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Molina, J.-M.; Real, M.; Jimenez-Diaz, I.; Belhassen, H.; Hedhili, A.; Torné, P.; Fernández, M.F.; Olea, N. Assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. Food Chem. Toxicol. 2014, 74, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhi, Y.; Xu, H.; Fang, H.; Jia, X. Zearalenone causes embryotoxicity and induces oxidative stress and apoptosis in differentiated human embryonic stem cells. Toxicol. In Vitro 2019, 54, 243–250. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C.A.; Cavaglieri, L.; Venâncio, A. Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef] [Green Version]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; GraslKraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 150. [Google Scholar]
- Abbès, S.; Ben Salah-Abbès, J.; Jebali, R.; Younes, R.B.; Oueslati, R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid bacteria. J. Immunotoxicol. 2016, 13, 46–54. [Google Scholar] [CrossRef]
- Hamid, A.S.; Tesfamariam, I.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention (Review). Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Xiulan, S.; Xiaolian, Z.; Jian, T.; Zhou, J.; Chu, F.S. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int. J. Food Microbiol. 2005, 99, 185–194. [Google Scholar] [CrossRef]
- Morales, H.; Sanchis, V.; Usall, J.; Ramos, A.J.; Marín, S. Effect of biocontrol agents Candida sake and Pantoea agglomerans on Penicillium expansum growth and patulin accumulation in apples. Int. J. Food Microbiol. 2008, 122, 61–67. [Google Scholar] [CrossRef]
- Jayashree, G.V.; Krupashree, K.; Rachitha, P.; Khanum, F. Patulin Induced Oxidative Stress Mediated Apoptotic Damage in Mice, and its Modulation by Green Tea Leaves. J. Clin. Exp. Hepatol. 2017, 7, 127–134. [Google Scholar] [CrossRef]
- Terezinha, F.; Melo, D.; Marques, I.; Greggio, S.; Dacosta, J.C.; Guecheva, T.N.; Saffi, J.; Rosa, R.M. DNA damage in organs of mice treated acutely with patulin, a known mycotoxin. Food Chem. Toxicol. 2012, 50, 3548–3555. [Google Scholar]
- Saxena, N.; Ansari, K.M.; Kumar, R.; Chaudhari, B.P.; Dwivedi, P.D.; Das, M. Role of mitogen activated protein kinases in skin tumorigenicity of Patulin. Toxicol. Appl. Pharmacol. 2011, 257, 264–271. [Google Scholar] [CrossRef]
- Drusch, S.; Kopka, S.; Kaeding, J. Stability of patulin in a juice-like aqueous model system in the presence of ascorbic acid. Food Chem. 2007, 100, 192–197. [Google Scholar] [CrossRef]
- De Curtis, F.; de Felice, D.V.; Ianiri, G.; De Cicco, V.; Castoria, R. Environmental factors affect the activity of biocontrol agents against ochratoxigenic Aspergillus carbonarius on wine grape. Int. J. Food Microbiol. 2012, 159, 17–24. [Google Scholar] [CrossRef]
- Paradells, S.; Rocamonde, B.; Llinares, C.; Garcia-verdugo, J.M.; Zipancic, I.; Miguel, J. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J. Apply Toxicol. 2015, 737–751. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Peng, Z.; Chen, L.; Zhang, W.; Nüssler, A.K.; Shi, S.; Liu, L.; Yang, W. Food raw materials and food production occurrences of deoxynivalenol in different regions. Trends Food Sci. Technol. 2019, 83, 41–52. [Google Scholar] [CrossRef]
- Feizollahi, E.; Iqdiam, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains. Appl. Sci. 2020, 10, 3530. [Google Scholar] [CrossRef]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol Decontamination in Raw and Germinating Barley Treated by Plasma-Activated Water and Intense Pulsed Light. Food Bioprocess Technol. 2019, 12, 246–254. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Dong, F.; Shi, J.; Xu, J. Esterase activity inspired selection and characterization of zearalenone degrading bacteria Bacillus pumilus ES-21. Food Control 2017, 77, 57–64. [Google Scholar] [CrossRef]
- Abbasian, N.; Momtaz, S.; Baeeri, M.; Navaei-nigjeh, M. Molecular and biochemical evidence on the role of zearalenone in rat polycystic ovary. Toxicon 2018, 154, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebra fish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Devreese, M.; De Baere, S.; Martel, A.; Van Immerseel, F.; Croubels, S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chuturgoon, A.; Phulukdaree, A.; Moodley, D. Fumonisin B1 induces global DNA hypomethylation in HepG2 cells—An alternative mechanism of action. Toxicology 2014, 315, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Alpertunga, B.; Ozden, S. Role of fumonisin B1 on DNA methylation changes in rat kidney and liver cells. Pharm. Biol. 2015, 53, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, G.I.; Shafee, M.; Samad, A.; Masood, H.; Khan, S.A. Biocontrol of Aflatoxin through Biodegradation by Using Environment Friendly Microbes. Pol. J. Environ. Stud. 2022, 31, 4985–4988. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Jin, Y.; Wu, C.; Shen, D.; Zhang, S.; Zhou, R. Aflatoxin B1 degradation by salt tolerant Tetragenococcus halophilus CGMCC 3792. Food Chem. Toxicol. 2018, 121, 430–436. [Google Scholar] [CrossRef]
- Teniola, O.; Addo, P.; Brost, I.; Farber, P.; Jany, K.; Alberts, J.; Vanzyl, W.; Steyn, P.; Holzapfel, W. Degradation of aflatoxin B1 by cell-free extracts of Rhodococcus erythropolis and Mycobacterium fluoranthenivorans sp. nov. DSM44556. Int. J. Food Microbiol. 2005, 105, 111–117. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Inês, A.; Rodrigues, A.I.; Guimarães, A.; Pereira, V.L.; Parpot, P.; Mendes-Faia, A.; Venâncio, A. Biodegradation of ochratoxin A by Pediococcus parvulus isolated from Douro wines. Int. J. Food Microbiol. 2014, 188, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmüller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef]
- Wochner, K.F.; Moreira, M.C.C.; Kalschne, D.L.; Drunkler, D.A. Detoxification of Aflatoxin B1 and M1 by Lactobacillus acidophilus and prebiotics in whole cow’s milk. J. Food Saf. 2019, 39, e12670. [Google Scholar] [CrossRef]
- Marina, S.; Sajid, M.; Yahong, Y.; Tianli, Y. Mycotoxin patulin in food matrices: Occurrence and its biological degradation strategies. Drug Metab. Rev. 2019, 51, 105–120. [Google Scholar]
- Tan, H.; Zhang, Z.; Hu, Y.; Wu, L.; Liao, F.; He, J.; Luo, B.; He, Y.; Zuo, Z.; Ren, Z.; et al. Isolation and characterization of Pseudomonas otitidis TH-N1 capable of degrading zearalenone. Food Control 2015, 47, 285–290. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Tang, Y.; Ma, Q.; Zhang, J.; Ji, C.; Zhao, L. Characterization and Genome Analysis of a Zearalenone—Degrading Bacillus velezensis Strain ANSB01E. Curr. Microbiol. 2019, 77, 273–278. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Zhang, D.; Zhao, M.; Zheng, D.; Lyu, Y.; Cheng, W.; Guo, P.; Cui, Z. Effective degradation of aflatoxin B1 using a novel thermophilic microbial consortium TADC7. Bioresour. Technol. 2017, 224, 166–173. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Farzaneh, M.; Shi, Z.-Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control 2012, 23, 100–106. [Google Scholar] [CrossRef]
- Topcu, A.; Bulat, T.; Wishah, R.; BoyacI, I.H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Zhang, D.; Zhao, M.; Zheng, D.; Peng, M.; Cheng, W.; Guo, P.; Cui, Z. Simultaneous degradation of aflatoxin B1 and zearalenone by a microbial consortium. Toxicon 2018, 146, 69–76. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT—Food Sci. Technol. 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zavistanaviciute, P.; Lele, V.; Ruzauskas, M.; Bartkevics, V.; Bernatoniene, J.; Gallo, P.; Tenore, G.C.; Santini, A. Lactobacillus plantarum LUHS135 and paracasei LUHS244 as functional starter cultures for the food fermentation industry: Characterisation, mycotoxin-reducing properties, optimisation of biomass growth and sustainable encapsulation by using dairy by-produc. LWT 2018, 93, 649–658. [Google Scholar] [CrossRef]
- Zheng, R.; Qing, H.; Ma, Q.; Huo, X.; Huang, S.; Zhao, L.; Zhang, J.; Ji, C. A Newly Isolated Alcaligenes faecalis ANSA176 with the Capability of Alleviating Immune Injury and Inflammation through Efficiently Degrading Ochratoxin A. Toxins 2022, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- Hormisch, D.; Brost, I.; Kohring, G.-W.; Giffhorn, F.; Kroppenstedt, R.; Stackebradt, E.; Färber, P.; Holzapfel, W. Mycobacterium fluoranthenivorans sp. nov., a Fluoranthene and Aflatoxin B1 Degrading Bacterium from Contaminated Soil of a Former Coal Gas Plant. Syst. Appl. Microbiol. 2004, 27, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Mwakinyali, S.E.; Ming, Z.; Xie, H.; Zhang, Q.; Li, P. Investigation and Characterization of Myroides odoratimimus Strain 3J2MO Aflatoxin B1 Degradation. J. Agric. Food Chem. 2019, 67, 4595–4602. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Mavumengwana, V. Degradation and detoxification of AFB1 by Staphylocococcus warneri, Sporosarcina sp. and Lysinibacillus fusiformis. Food Control 2016, 68, 92–96. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Sidu, S.; Adebiyi, J.A.; Mavumengwana, V. Aflatoxin B1 degradation by culture and lysate of a Pontibacter specie. Food Control 2017, 80, 99–103. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Ramadan, M.F.; El-Gohery, S.S.; Abol-Ela, M.F.; Azeke, M.A. Ability of selected microorganisms for removing aflatoxins invitro and fate of aflatoxins in contaminated wheat during baladi bread baking. Food Control 2013, 33, 287–292. [Google Scholar] [CrossRef]
- Alberts, J.F.; Engelbrecht, Y.; Steyn, P.S.; Holzapfel, W.H.; van Zyl, W.H. Biological degradation of aflatoxin B1 by Rhodococcus erythropolis cultures. Int. J. Food Microbiol. 2006, 109, 121–126. [Google Scholar] [CrossRef]
- Mosallaie, F.; Jooyandeh, H.; Hojjati, M.; Fazlara, A. Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci. Biotechnol. 2020, 29, 793–803. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Zhu, Z.; Ji, F.; Yin, X.; Hong, Q.; Shi, J. Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the degradation of Zearalenone by Bacillus spp. Food Control 2016, 68, 244–250. [Google Scholar] [CrossRef]
- He, W.-J.; Yuan, Q.-S.; Zhang, Y.-B.; Guo, M.-W.; Gong, A.-D.; Zhang, J.-B.; Wu, A.-B.; Huang, T.; Qu, B.; Li, H.-P.; et al. Aerobic De-Epoxydation of Trichothecene Mycotoxins by a Soil Bacterial Consortium Isolated Using In Situ. Toxins 2016, 8, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokoena, M.P.; Chelule, P.K. Reduction of Fumonisin B1 and Zearalenone by Lactic Acid Bacteria in Fermented Maize Meal. J. Food Prot. 2005, 68, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Malaphan, W.; Xing, F.; Yu, B. Biodetoxification of fungal mycotoxins zearalenone by engineered probiotic bacterium Lactobacillus reuteri with surface-displayed lactonohydrolase. Appl. Microbiol. Biotechnol. 2019, 103, 8813–8824. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Horváth, E.; Peles, F.; Pócsi, I.; Miklós, I. Insight into Yeast—Mycotoxin Relations. Agriculture 2021, 11, 1291. [Google Scholar] [CrossRef]
- Cao, H.; Liu, D.; Mo, X.; Xie, C.; Yao, D. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiol. Res. 2011, 166, 475–483. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, J.; Solairaj, D.; Fu, Y.; Zhang, H. Efficacy of Meyerozyma guilliermondii in controlling patulin production by Penicillium expansum in shuijing pears. Biol. Control 2022, 168, 104856. [Google Scholar] [CrossRef]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 2004, 97, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Petruzzi, L.; Bevilacqua, A.; Baiano, A.; Beneduce, L.; Corbo, M.R.; Sinigaglia, M. Study of Saccharomyces cerevisiae W13 as a functional starter for the removal of ochratoxin A. Food Control 2014, 35, 373–377. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Franco, L.T.; Rottinghaus, G.E.; Kobashigawa, E.; Ledoux, D.R.; Daković, A.; Oliveira, C.A. In vitro evaluation of the ability of beer fermentation residue containing Saccharomyces cerevisiae to bind mycotoxins. Food Res. Int. 2015, 77, 643–648. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Pizzolitto, R.P.; Salvano, M.A.; Dalcero, A.M. Analysis of fumonisin B1 removal by microorganisms in co-occurrence with aflatoxin B1 and the nature of the binding process. Int. J. Food Microbiol. 2012, 156, 214–221. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Ponsone, M.L.; Chiotta, M.L.; Combina, M.; Dalcero, A.; Chulze, S. Biocontrol as a strategy to reduce the impact of ochratoxin A and Aspergillus section Nigri in grapes. Int. J. Food Microbiol. 2011, 151, 70–77. [Google Scholar] [CrossRef]
- Fiori, S.; Paolo, P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. International Journal of Food Microbiology Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Meca, G.; Ritieni, A.; Zhou, T.; Li, X.Z.; Mañes, J. Degradation of the minor Fusarium mycotoxin beauvericin by intracellular enzymes of Saccharomyces cerevisiae. Food Control 2013, 33, 352–358. [Google Scholar] [CrossRef]
- Zhu, R.; Feussner, K.; Wu, T.; Yan, F.; Karlovsky, P.; Zheng, X. Detoxification of mycotoxin patulin by the yeast Rhodosporidium paludigenum. Food Chem. 2015, 179, 1–5. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Jin, Y.; Wu, C.; Shen, D.; Zhang, S.; Zhou, R. Mechanism and kinetics of degrading aflatoxin B1 by salt tolerant Candida versatilis CGMCC 3790. J. Hazard. Mater. 2018, 359, 382–387. [Google Scholar] [CrossRef]
- Varga, J.; Péteri, Z.; Tábori, K.; Téren, J.; Vágvölgyi, C. Degradation of ochratoxin A and other mycotoxins by Rhizopus isolates. Int. J. Food Microbiol. 2005, 99, 321–328. [Google Scholar] [CrossRef]
- Bdswald, C.; Engelhardt, G.; Vogel, H.; Wallnofer, P.R. Metabolism of the Fusatium Mycotoxins Zearalenone and Deoxynivalenol by Yeast Strains of Technological Relevance. Nat. Toxins 1995, 3, 138–144. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Cimmarusti, M.T.; Mirabelli, V.; Haidukowski, M.; Logrieco, A.F.; Caliandro, R.; Mule, G. In vitro single and combined mycotoxins degradation by Ery4 laccase from Pleurotus eryngii and redox mediators. Food Control 2018, 90, 401–406. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2011, 314, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zeinvand-Lorestani, H.; Sabzevari, O.; Setayesh, N.; Amini, M.; Nili-Ahmadabadi, A.; Faramarzi, M.A. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wu, Z.; Wu, S.; Dai, Y.; Sun, C. Degradation of ochratoxin A by Bacillus amyloliquefaciens ASAG1. Food Addit. Contam. Part A 2015, 32, 564–571. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Santos, L.; Venâncio, A. Degradation of ochratoxin A by proteases and by a crude enzyme of Aspergillus niger. Food Biotechnol. 2006, 20, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Liu, B.; Wang, Z.; Han, C.; Meng, X.; Zhang, F. Effective degradation of the mycotoxin patulin in pear juice by porcine pancreatic lipase. Food Chem. Toxicol. 2019, 133, 110769. [Google Scholar] [CrossRef]
- Tang, H.; Li, X.; Zhang, F.; Meng, X.; Liu, B. Biodegradation of the mycotoxin patulin in apple juice by Orotate phosphoribosyltransferase from Rhodotorula mucilaginosa. Food Control 2019, 100, 158–164. [Google Scholar] [CrossRef]
- Alberts, J.F.; Gelderblom, W.C.A.; Botha, A.; van Zyl, W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef]
- Afsharmanesh, H.; Perez-Garcia, A.; Zeriouh, H.; Ahmadzadeh, M.; Romero, D. Aflatoxin degradation by Bacillus subtilis UTB1 is based on production of an oxidoreductase involved in bacilysin biosynthesis. Food Control 2018, 94, 48–55. [Google Scholar] [CrossRef]
- Samuel, M.S.; Sivaramakrishna, A.; Mehta, A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int. Biodeterior. Biodegrad. 2014, 86, 202–209. [Google Scholar] [CrossRef]
- Ianiri, G.; Pinedo, C.; Fratianni, A.; Panfili, G.; Castoria, R. Patulin degradation by the biocontrol yeast Sporobolomyces sp. Is an inducible process. Toxins 2017, 9, 61. [Google Scholar] [CrossRef]
- Young, J.C.; Zhou, T.; Yu, H.; Zhu, H.; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef]
- Koch, M.; Maul, R.; Brodehl, A.; Anne, M. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In Vitro Transformation of the Fusarium Mycotoxins Deoxynivalenol and ZearaLenone by the Normal Gut Microflora of Pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef]
- Péteri, Z.; Téren, J.; Vágvölgyi, C.; Varga, J. Ochratoxin degradation and adsorption caused by astaxanthin-producing yeasts. Food Microbiol. 2007, 24, 205–210. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhang, H.; Apaliya, M.T.; Zhang, X.; Zhao, L.; Jiang, Z.; Yang, Q.; Gu, X. Identification and toxicological analysis of products of patulin degradation by Pichia caribbica. Biol. Control 2018, 123, 127–136. [Google Scholar] [CrossRef]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Elsanhoty, R.M.; Salam, S.A.; Ramadan, M.F.; Badr, F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control 2014, 43, 129–134. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Tabatabaie Yazdi, F.; Shabani, A.A.; Mortazavi, S.A.; Marvdashti, L.M. Aflatoxin binding efficiency of Saccharomyces cerevisiae mannoprotein in contaminated pistachio nuts. Food Control 2018, 87, 17–21. [Google Scholar] [CrossRef]
- Alekinejad, H.M.; Franciscus, R.; Akker, M.A.A.S.; Remmels, J.F.I.N.K. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-F.; Zhou, X.-W.; Wang, F.; Shen, Y.-D.; Xiao, Z.-L.; Zhang, S.-W.; Li, Y.-J.; Wang, H. Development of a Monoclonal Antibody-Based ELISA for the Detection of Alternaria Mycotoxin Tenuazonic Acid in Food Samples. Food Anal. Methods 2020, 13, 1594–1602. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, S.; Gao, X.; Ma, Q.; Lei, Y.; Bai, X.; Ji, C. Preparation, purification and characteristics of an aflatoxin degradation enzyme from Myxococcus fulvus ANSM068. J. Appl. Microbiol. 2010, 110, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Motomura, M.; Toyomasu, T.; Mizuno, K.; Shinozawa, T. Purification and characterization of an aflatoxin degradation enzyme from Pleurotus ostreatus. Microbiol. Res. 2003, 158, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Peng, X.; Li, X.; Meng, X.; Liu, B. Biodegradation of mycotoxin patulin in apple juice by calcium carbonate immobilized porcine pancreatic lipase. Food Control 2018, 88, 69–74. [Google Scholar] [CrossRef]
- Medina, A.; Mohale, S.; Samsudin, N.I.P.; Rodriguez-Sixtos, A.; Rodriguez, A.; Magan, N. Biocontrol of mycotoxins: Dynamics and mechanisms of action. Curr. Opin. Food Sci. 2017, 17, 41–48. [Google Scholar] [CrossRef]
- Illueca, F.; Vila-Donat, P.; Calpe, J.; Luz, C.; Meca, G.; Quiles, J.M. Antifungal activity of biocontrol agents in vitro and potential application to reduce mycotoxins (Aflatoxin B1 and ochratoxin A). Toxins 2021, 13, 752. [Google Scholar] [CrossRef]
- Zapasnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Yao, M.; Wu, J.; Li, B.; Xiao, H.; McClements, D.J.; Li, L. Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocoll. 2017, 72, 228–236. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, M.; Wang, Y.; Wu, H.; Xiong, L.; Sun, Q. Enhanced viability of layer-by-layer encapsulated Lactobacillus pentosus using chitosan and sodium phytate. Food Chem. 2019, 285, 260–265. [Google Scholar] [CrossRef]
- Alehosseini, A.; Sarabi-jamab, M.; Ghorani, B.; Kadkhodaee, R. Electro-encapsulation of Lactobacillus casei in high-resistant capsules of whey protein containing transglutaminase enzyme. LWT—Food Sci. Technol. 2019, 102, 150–158. [Google Scholar] [CrossRef]
- Tapingkae, W.; Srinual, O.; Lumsangkul, C.; Van Doan, H.; Chiang, H.-I.; Manowattana, A.; Boonchuay, P.; Chaiyaso, T. Industrial-Scale Production of Mycotoxin Binder from the Red Yeast Sporidiobolus pararoseus KM281507. J. Fungi 2022, 8, 353. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef]
- Chen, W.C.; Chiou, T.Y.; Delgado, A.L.; Liao, C.S. The control of rice blast disease by the novel biofungicide formulations. Sustainability 2019, 11, 3449. [Google Scholar] [CrossRef] [Green Version]
- Zamoum, M.; Allali, K.; Benadjila, A.; Zitouni, A.; Goudjal, Y. Formulation of biofungicides based on Streptomyces lincolnensis strain SZ03 spores and efficacy against R. solani damping-off of tomato seedlings. ResearchSquare 2022, 1–22. [Google Scholar] [CrossRef]
- Mousumi Das, M.; Aguilar, C.N.; Haridas, M.; Sabu, A. Production of bio-fungicide, Trichoderma harzianum CH1 under solid-state fermentation using coffee husk. Bioresour. Technol. Rep. 2021, 15, 100708. [Google Scholar] [CrossRef]
- Sudantha, I.M.; Suwardji, S. Trichoderma biofungicides formulations on shallot growth, yield and fusarium wilt disease resistance. IOP Conf. Ser. Earth Environ. Sci. 2021, 824, 12032. [Google Scholar] [CrossRef]
- Souza, P.M.D.; Magalhães, P.D.O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]



| Toxins | Effects | Fungi Producer | WHO Recommendation | References |
|---|---|---|---|---|
| Aflatoxin B1 | Cancerogenic, teratogenic, mutagenic | Aspergillus flavus, Aspergillus parasiticus, Aspergillus nominus, and Aspergillus niger | 15 µg/kg in peanuts | [5,30,31,39,78,79,80] |
| Patulin | Genotoxicity, mutagenicity, gastrointestinal disorders, edema | Penicillium, Byssochlamys, and Aspergillus species | 50 µg/kg in apple juice | [27,81,82,83,84,85] |
| OTA | Nephrotoxic and neurotoxic effects, affects mammary functions | Aspergillus ochraceus, Penicillium verrucosum, Aspergillus carbonarius, and Aspergillus niger | 5 µg/kg in wheat and barley | [50,52,54,86,87] |
| DON | Intestinal damage, emetic effects, immune-toxic | Fusarium graminearum and Fusarium culmorum | 2000 µg/kg in wheat, barley, and maize | [88,89,90] |
| ZEN | Cytogenetic toxicity, decreases fertility, embryotoxicity, immunotoxicity, estrogenic, anti-androgenic activities | Fusarium graminearum, Fusarium culmorum, Fusarium cerealis, Fusarium equiseti, and Fusarium semitectum | TDI1 0.25 µg/kg by EFSA2 | [70,71,72,73,74,91,92,93] |
| Fumonisin B1 | Neurotoxicity, Hepatotoxicity, nephrotoxicity | Fusarium verticilloides and Fusarium proliferatum | Total of FB1 + FB2: 2000 µg/kg in maize flour and maize meal | [59,94,95,96] |
| Bacteria | Medium Culture | Main Effects | References |
|---|---|---|---|
| Bacillus subtilis UTBSP1 | (1) Nutrient broth culture (2) Pistachio nut | Detoxification of AFB1 by 85.66% and 95%, respectively, in the nutrient broth culture and the pistachio nuts in optima conditions of 35–40 °C during 24 h. | [109] |
| Mycobacterium fluoranthenivorans sp. | Medium culture with AFB1 | The AFB1 concentration was reduced by 70% to 80% within 36 h. | [115] |
| Myroides odoratimimus strain 3J2MO | Medium culture with AFB1 | Degradation of 93.82% of the AFB1 after incubation for 48 h at 37 °C. | [116] |
| Pseudomonas fluorescens strain 3JW1 | (1) Medium culture with AFB1 (2) Peanut medium (3) Peanut kernels | Degradation of AFB1 by 88.3% in 96 h. | [18] |
| Rhodococcus pyridinivorans K408 | Bioethanol produced by Aspergillus flavus-contaminated corn | Degradation rate was more than 63% in the solid phase and 75% in the liquid phase after 12 experiment days. | [26] |
| Staphylococcus warneri, Sporosarcina sp., Lysinibacillus fusiformis | Medium culture with AFB1 standard | Both cultures and lysates degraded AFB1, and the addition of a protease inhibitor enhanced the degradation rate of the lysate. | [117] |
| Enterococcus faecium M74 and EF031 strains | Medium culture with FB1 solution | AFB1 removal by 19.3 to 30.5% for M74 strain and 23.4 to 37.5% for EF031 strain. | [110] |
| Pontibacter specie | Medium culture with aflatoxin B1 standard | Lysates and cultures both degraded AFB1. | [118] |
| Microbial consortium, TADC7 | Medium culture with aflatoxin B1 standard | Degradation of more than 95% of the amount of AFB1 after five days cultivation in PCS medium at 55 °C. | [107] |
| Lacticaseibacillus rhamnosus (previously Lactobacillus rhamnosus) strains LBGG and LC705 | Medium culture with aflatoxin B1 standard | A rapid removal of 80% of AFB1 by both two strains. | [108] |
| Lacticaseibacillus rhamnosus (previously Lactobacillus rhamnosus) TISTR 541 | Bread produced by contaminated wheat flour | Decrease in AFB1 levels during mixing and fermentation process. | [119] |
| Rhodococcus erythropolis | Medium culture with aflatoxin B1 standard | A significant reduction in the amount of AFB1 when treated with the Rhodococcus erythropolis extracellular extracts. | [120] |
| Lactobacillus acidophilus and prebiotics | Whole cow’s milk | Reduction in AFB1 of 13.53 to 35.53%. | [103] |
| Lactobacillus acidophilus and Lacticaseibacillus (previously Lactobacillus rhamnosus) | Yogurt samples | Binding of AFB1 by 64.56 to 96.58% during 21 days of storage. | [121] |
| Bacteria | Toxins | Medium Culture | Main Effects | References |
|---|---|---|---|---|
| Enterococcus faecium M74 and EF031 strains | Patulin | Medium culture enriched with patulin solution | Patulin removal of 15.8 to 41.6% for M74 strain and 19.5 to 45.3% for EF031 strain. | [110] |
| Bacillus pumilus ES-21 | Zearalenone | Medium culture with ZEN standard | The degradation rate was more than 95.7%. | [91] |
| Bacillus amyloliquefaciens ZDS-1 | Zearalenone | (1) Medium culture with ZEN standard (2) Contaminated wheat samples | ZEN degradation with a concentration ranging from 1 mg/L to 100 mg/L for specific optimal conditions, which are temperature 30 °C, pH from 6.0 to 7.0, and a microorganism concentration of 5.1 × 108 CFU/mL. | [122] |
| Rhodococcus pyridinivorans strains (K408 and AK37) | AFB1, T2 toxin, ZEA | Medium culture with mycotoxin standard solutions | Degradation of the 03 mycotoxins and increase in the ZON degradation capacity from 60% to 95% in the multi-mycotoxin degradation system | [22] |
| Microbial consortium TADC7 | AFB1, ZEN | Medium culture with mycotoxin standard solutions | Degradation of AFB1 by 98.9% and ZEN by 88.5% after 168 h. | [111] |
| Pseudomonas otitidis TH-N1 | Zearalenone | Medium culture with a ZEN standard | Degradation of ZEN under optimal conditions: Temperature 37 °C, pH 4 to pH 5, and bacterial concentration of 109 CFU/mL. | [105] |
| Bacterial consortium PGC-3 | DON, NIV | Medium culture with mycotoxin standard solution | Biotransformation of DON into de-epoxy-DON and NIV into de-epoxy-NIV with optimal conditions of pH 5–10 and temperatures of 20–37 °C in aerobic conditions. | [123] |
| Lactic acid bacteria | DON, T-2, HT-2, ZEN | Malting wheat | Reduction in the amount of DON, T-2, HT-2, and ZEN of, respectively, 23%, 34%, 58%, and 73% in malting wheat samples. | [112] |
| Lactic acid bacteria | FB1, ZEN | Maize meal | Reduction in ZEN of 68.3% and FB1 of 75% after 4 incubation days. | [124] |
| Limosilactobacillus reuteri (previously Lactobacillus reuteri) | ZEN | Nutrient broth and maize kernels | Hydrolysis of 5.0 mg/L ZEN for 8 h in nutrient broth and hydrolysis of 2.5 mg/kg ZEN for 4 h in ZEN-contaminated maize kernels. | [125] |
| Bacillus velezensis Strain ANSB01E | ZEN | Liquid medium and moldy corn | ZEN degradation of 95% in the liquid medium and of 25% in the moldy corn after 48 h. | [106] |
| Yeasts | Toxins | Medium Culture | Main Effects | References |
|---|---|---|---|---|
| Saccharomyces cerevisiae | Beauvericin (BEA) | (1) Standard of BEA (2) Corn flour | In total, 89.1 to 99.3% degradation rate in the standard solution against 73.5 to 91% in the cornflour. | [137] |
| Rhodosporidium paludigenum | Patulin | Patulin standard | Removal of the total amount of patulin after two days at 28 °C. | [138] |
| Armillariella tabescens | Aflatoxin B1 | Aflatoxin B1 standard | Cleavage of the bis-furan ring. | [127] |
| Candida versatilis CGMCC 3790 | Aflatoxin B1 | A mixture of steamed soybean and baked wheat flour | Degradation dependent on initial AFB1 concentration. | [139] |
| Rhizopus stolonifer | OTA | Wheat contaminated by OTA | Degradation of 96.5% of OTA. | [140] |
| Candida intermedia, Lachancea thermotolerans, Candida friedrichii | OTA | Grape juice | Reductions in OTA by Candida intermedia, Lachancea thermotolerans, Candida friedrichii of 73%, 75%, and 70%, respectively. | [136] |
| Candida tropicalis, Torulaspora delbriickii, Zygosaccharomyces rouxii, and Saccharomyces strains | ZEN | Growth media | Biodegradation of ZEN into α- zearalenol and β-zearalenol. | [141] |
| Saccharomyces cerevisiae W13 | OTA | Semi-synthetic medium | Removal of an amount of OTA from 6 to 57.21% with the highest level obtained at 30 °C with 250 g/L of sugar. | [130] |
| Toxins | Medium | Enzymes | Main Effects | References |
|---|---|---|---|---|
| Patulin | Apple juice | Orotate phosphoribosyltransferase | The degradation rate can reach over 80%. | [148] |
| Aflatoxin B1 | Medium culture with aflatoxin B1 standard | Aflatoxin-oxidase (AFO) | Cleavage of the bis-furan ring. | [127] |
| Aflatoxin B1 | Citrate buffer solution containing 20% DMSO | Laccase | Under optimal conditions, which are a temperature of 35 °C, a pH of 4.5, and a laccase activity of 30 U/mL, 67% of the AFB1 total amount was degraded after two days. | [144] |
| OTA | LB medium | Carboxypeptidase from Bacillus amyloliquefaciens ASAG1 | Decrease of 41% and 72%, respectively, when cultivated with the supernatant and the purified protein of carboxypeptidase. | [145] |
| OTA | Buffer systems with enzymes | Commercial protease A, commercial pancreatin, and an enzyme extract isolated from Aspergillus niger MUM | At pH 7.5 and 37 °C, protease A and pancreatin reduce the OTA level, respectively, by 87.3%, 43.4%, and 99.8% after 25 h. | [146] |
| AFB1 | Reaction mixture | Manganese protease MnP | In total, 86% of AFB1 levels decrease after 48 h and 5 nkat of MnP. | [143] |
| Patulin | Pear juice | Porcine pancreatic lipase (PPL) | Patulin degradation with 0.02 g/mL PPL and 0.375 mg/L of PAT at 40 °C within 24 h. | [147] |
| Aflatoxin B1, Fumonisin B1, Ochratoxin A, Zearalenone, T-2 | Medium culture with a standard solution of mycotoxins | Ery4 laccase from Saccharomyces cerevisiae | AFB1, FB1, OTA, ZEN, and T-2 toxin degradations of 73%, 74%, 27%, 100%, and 40%, respectively. | [142] |
| Aflatoxin B1 | Medium culture with aflatoxin B1 standard | Laccase from white rot fungi | In total, 40.45% degradation of AFB1 by Peniophora sp. SCC0152; 35.90% degradation of AFB1 by Pleurotus ostreatus St2; 3; 87.34% degradation of AFB1 by pure laccase from Trametes versicolor. | [149] |
| Microorganisms | Genes or Enzymes | Toxins | Degradation Reactions | Obtained Metabolites | References |
|---|---|---|---|---|---|
| Tetragenococcus halophilus | Enzyme ND * | Aflatoxin B1 | Adsorption + enzymatical action | C14H10O4, C18H16O8, C14H12O3, C16H20O4, C14H16O2, C14H20O2 | [99] |
| Candida versatilis CGMCC 3790 | Enzyme ND | Aflatoxin B1 | Adsorption + enzymatical action | C14H10O4, C14H12O3, C13H12O2, C11H10O4. | [139] |
| PhanerochaetesordidaYK-624 | Manganese protease MnP | Aflatoxin B1 | Oxidation + hydrolysis | AFB1-8,9-dihydrodiol | [143] |
| Rhodosporidium paludigenum | Enzyme ND | Patulin | ND | Desoxypatulinic acid | [138] |
| Bacillus pumilus ES-21 | Esterase | Zearalenone (ZEN) | Cleavage of the lactone ring, followed by des-carboxylation. The enzymatic process follows first-order kinetics with t1/2 of 6.52 h. | 1-(3,5-dihydroxyphenyl)-60-hydroxy-l0- undecen-l00-one | [91] |
| Eggerthella sp. | Enzyme ND | DON, HT-2, T-2 triol and T-2 tetraol | De-epoxidation | De-epoxy- DON, de-epoxy T-2triol, de-epoxy HT-2, de-epoxy T-2 tetraol for the 04 parents in different ratios | [62] |
| Saccharomyces cerevisiae | Endo and Exo enzymes synthesized by the yeast during the fermentation | Patulin | The mechanism was enzymatical and the production of the relevant PAT-metabolizing enzymes synthesized by the yeast cells is not induced by PAT preincubation | E-ascladiol | [27] |
| Sporobolomyces sp. IAM 13481 | ND | Patulin | The mechanism was induced by pretreatment with patulin. | DPA and (Z)-ascladiol | [152] |
| Pediococcus parvulus UTAD | Peptidases | OTA | Hydrolysis of the OTA amide group. | Otα | [101] |
| Rhizopus and Aspergillus species | ZEN | Glycosylation, sulfate-conjugation | ZEN-14-sulfate, ZEN-O-14, ZEN- O-16-glucoside, α-zearalenol, α- zearalenol-sulfate | [154] | |
| Phaffia rhodozyma | Metalloprotease | OTA | ND | Otα | [156] |
| Gut Microflora of Pigs | ND | DON | De-epoxidation | De-epoxy-DON | [155] |
| Gut Microflora of Pigs | ND | ZEN | Hydrolyze | α-zearalenol | [155] |
| Bacillus subtilis UTB1 | Gene bacC, | AFB1 | Reduction in the double bond Hydrolysis of the ester bond Des-carboxylation | AFD1 | [150] |
| Pseudomonas putida | ND | AFB1 | Opening of the lactone ring | AFD1, AFD2, AFD3 | [151] |
| Pichia caribbica | Intracellular enzymes | Patulin | Unidentified | Ascladiol and unknown compound | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins 2022, 14, 729. https://doi.org/10.3390/toxins14110729
Ndiaye S, Zhang M, Fall M, Ayessou NM, Zhang Q, Li P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins. 2022; 14(11):729. https://doi.org/10.3390/toxins14110729
Chicago/Turabian StyleNdiaye, Seyni, Minhui Zhang, Mouhamed Fall, Nicolas M. Ayessou, Qi Zhang, and Peiwu Li. 2022. "Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications" Toxins 14, no. 11: 729. https://doi.org/10.3390/toxins14110729
APA StyleNdiaye, S., Zhang, M., Fall, M., Ayessou, N. M., Zhang, Q., & Li, P. (2022). Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins, 14(11), 729. https://doi.org/10.3390/toxins14110729