Targeted Sphingolipid Analysis in Heart, Gizzard, and Breast Muscle in Chickens Reveals Possible New Target Organs of Fumonisins
Abstract
:1. Introduction

2. Results
2.1. Effect of Fumonisins According to the Class of Sphingolipids
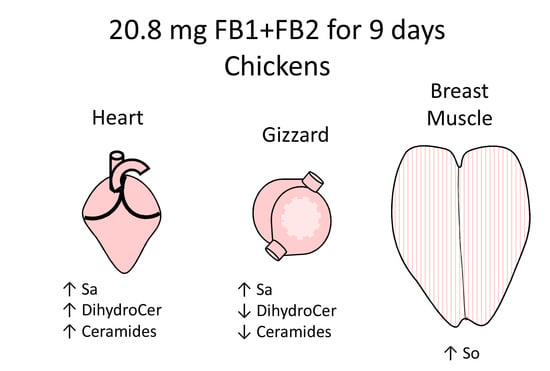
2.2. Effects of Fumonisins on Sphingolipids in Heart
2.3. Effects of Fumonisins on Sphingolipids in Gizzard
2.4. Effects of Fumonisins on Sphingolipids in Breast Muscle
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Analytes and Reagents
5.2. Tissue Samples
5.3. Sphingolipids in Tissues
5.4. Analytes and Reagents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA; Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for Animal Health Related to the Presence of Fumonisins, Their Modified Forms and Hidden Forms in Feed. EFSA J. 2018, 16, e05242. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.-C.; Nielsen, E.; et al. Assessment of Information as Regards the Toxicity of Fumonisins for Pigs, Poultry and Horses. EFSA J. Eur. Food Saf. Auth. 2022, 20, e07534. [Google Scholar] [CrossRef]
- FUMONISINS (JECFA 47, 2001). Available online: http://www.inchem.org/documents/jecfa/jecmono/v47je03.htm (accessed on 18 March 2019).
- FDA. 2011. Available online: https://www.ngfa.org/wp-content/uploads/NGFAComplianceGuide-FDARegulatoryGuidanceforMycotoxins8-2011.pdf (accessed on 11 August 2016).
- IARC MOnograph 82, I.W.G. FUMONISIN B1. 2002. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono82.pdf (accessed on 6 November 2022).
- Liu, X.; Fan, L.; Yin, S.; Chen, H.; Hu, H. Molecular Mechanisms of Fumonisin B1-Induced Toxicities and Its Applications in the Mechanism-Based Interventions. Toxicon 2019, 167, 1–5. [Google Scholar] [CrossRef]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A.A. Molecular and Epigenetic Modes of Fumonisin B1 Mediated Toxicity and Carcinogenesis and Detoxification Strategies. Crit. Rev. Toxicol. 2021, 51, 76–94. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef] [PubMed]
- Wangia-Dixon, R.N.; Nishimwe, K. Molecular Toxicology and Carcinogenesis of Fumonisins: A Review. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2021, 39, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of Sphingolipid Biosynthesis by Fumonisins. Implications for Diseases Associated with Fusarium Moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide Synthase Inhibition by Fumonisins: A Perfect Storm of Perturbed Sphingolipid Metabolism, Signaling, and Disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.T.; Tardieu, D.; Auvergne, A.; Bailly, J.D.; Babilé, R.; Durand, S.; Benard, G.; Guerre, P. Serum Sphinganine and the Sphinganine to Sphingosine Ratio as a Biomarker of Dietary Fumonisins during Chronic Exposure in Ducks. Chem. Biol. Interact. 2006, 160, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, D.; Matard-Mann, M.; Collén, P.N.; Guerre, P. Strong Alterations in the Sphingolipid Profile of Chickens Fed a Dose of Fumonisins Considered Safe. Toxins 2021, 13, 770. [Google Scholar] [CrossRef]
- Guerre, P.; Travel, A.; Tardieu, D. Targeted Analysis of Sphingolipids in Turkeys Fed Fusariotoxins: First Evidence of Key Changes That Could Help Explain Their Relative Resistance to Fumonisin Toxicity. Int. J. Mol. Sci. 2022, 23, 2512. [Google Scholar] [CrossRef]
- Loiseau, N.; Polizzi, A.; Dupuy, A.; Therville, N.; Rakotonirainy, M.; Loy, J.; Viadere, J.-L.; Cossalter, A.-M.; Bailly, J.-D.; Puel, O.; et al. New Insights into the Organ-Specific Adverse Effects of Fumonisin B1: Comparison between Lung and Liver. Arch. Toxicol. 2015, 89, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Park, H.; Laviad, E.L.; Silva, L.C.; Lahiri, S.; Stiban, J.; Erez-Roman, R.; Brügger, B.; Sachsenheimer, T.; Wieland, F.; et al. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: I. Alterations in Lipid Metabolic Pathways. J. Biol. Chem. 2010, 285, 10902–10910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pewzner-Jung, Y.; Brenner, O.; Braun, S.; Laviad, E.L.; Ben-Dor, S.; Feldmesser, E.; Horn-Saban, S.; Amann-Zalcenstein, D.; Raanan, C.; Berkutzki, T.; et al. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: II. Insights into Molecular Changes Leading to Hepatopathy. J. Biol. Chem. 2010, 285, 10911–10923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, S.; Abel, S.; Burger, H.-M.; van der Westhuizen, L.; Swanevelder, S.; Gelderblom, W.C.A. Differential Modulation of the Lipid Metabolism as a Model for Cellular Resistance to Fumonisin B1–Induced Cytotoxic Effects in Vitro. Prostaglandins Leukot. Essent. Fat. Acids 2016, 109, 39–51. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Zhao, Y.; Zang, J.; Gao, H. Fumonisin B1 Exposure Induces Apoptosis of Human Kidney Tubular Epithelial Cells through Regulating PTEN/PI3K/AKT Signaling Pathway via Disrupting Lipid Raft Formation. Toxicon 2021, 204, 31–36. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Tso, K.-H.; Fan, Y.-K.; Chiang, H.-I.; Ju, J.-C. Mycotoxin Fumonisin B1 Interferes Sphingolipid Metabolisms and Neural Tube Closure during Early Embryogenesis in Brown Tsaiya Ducks. Toxins 2021, 13, 743. [Google Scholar] [CrossRef]
- Mignard, V.; Dubois, N.; Lanoé, D.; Joalland, M.-P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F.M.; Lalier, L. Sphingolipid Distribution at Mitochondria-Associated Membranes (MAMs) upon Induction of Apoptosis. J. Lipid Res. 2020, 61, 1025–1037. [Google Scholar] [CrossRef]
- Guerre, P.; Matard-Mann, M.; Nyvall Collén, P. Targeted Sphingolipid Analysis in Chickens Suggests Different Mechanisms of Fumonisin Toxicity in Kidney, Lung, and Brain. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 170, 113467. [Google Scholar] [CrossRef]
- Bowler, R.P.; Jacobson, S.; Cruickshank, C.; Hughes, G.J.; Siska, C.; Ory, D.S.; Petrache, I.; Schaffer, J.E.; Reisdorph, N.; Kechris, K. Plasma Sphingolipids Associated with Chronic Obstructive Pulmonary Disease Phenotypes. Am. J. Respir. Crit. Care Med. 2015, 191, 275–284. [Google Scholar] [CrossRef]
- Kovacic, B.; Sehl, C.; Wilker, B.; Kamler, M.; Gulbins, E.; Becker, K.A. Glucosylceramide Critically Contributes to the Host Defense of Cystic Fibrosis Lungs. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2017, 41, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Berdyshev, E.V.; Mikosz, A.M.; Bronova, I.A.; Bronoff, A.S.; Jung, J.P.; Beatman, E.L.; Ni, K.; Cao, D.; Scruggs, A.K.; et al. Role of Glucosylceramide in Lung Endothelial Cell Fate and Emphysema. Am. J. Respir. Crit. Care Med. 2019, 200, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Alessenko, A.V.; Albi, E. Exploring Sphingolipid Implications in Neurodegeneration. Front. Neurol. 2020, 11, 437. [Google Scholar] [CrossRef]
- Smith, G.W.; Constable, P.D.; Tumbleson, M.E.; Rottinghaus, G.E.; Haschek, W.M. Sequence of Cardiovascular Changes Leading to Pulmonary Edema in Swine Fed Culture Material Containing Fumonisin. Am. J. Vet. Res. 1999, 60, 1292–1300. [Google Scholar]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin Toxicosis in Swine: An Overview of Porcine Pulmonary Edema and Current Perspectives. Environ. Health Perspect. 2001, 109 (Suppl. 2), 251–257. [Google Scholar] [CrossRef]
- Sharma, D.; Asrani, R.K.; Ledoux, D.R.; Rottinghaus, G.E.; Gupta, V.K. Toxic Interaction between Fumonisin B1 and Moniliformin for Cardiac Lesions in Japanese Quail. Avian Dis. 2012, 56, 545–554. [Google Scholar] [CrossRef]
- Terciolo, C.; Bracarense, A.P.; Souto, P.C.M.C.; Cossalter, A.-M.; Dopavogui, L.; Loiseau, N.; Oliveira, C.A.F.; Pinton, P.; Oswald, I.P. Fumonisins at Doses below EU Regulatory Limits Induce Histological Alterations in Piglets. Toxins 2019, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Borodzicz-Jażdżyk, S.; Jażdżyk, P.; Łysik, W.; Cudnoch-Jȩdrzejewska, A.; Czarzasta, K. Sphingolipid Metabolism and Signaling in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 915961. [Google Scholar] [CrossRef]
- Ledoux, D.R.; Brown, T.P.; Weibking, T.S.; Rottinghaus, G.E. Fumonisin Toxicity in Broiler Chicks. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 1992, 4, 330–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubena, L.F.; Edrington, T.S.; Kamps-Holtzapple, C.; Harvey, R.B.; Elissalde, M.H.; Rottinghaus, G.E. Influence of Fumonisin B1, Present in Fusarium Moniliforme Culture Material, and T-2 Toxin on Turkey Poults. Poult. Sci. 1995, 74, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Bunte, R.M.; Dombrink-Kurtzman, M.A.; Richard, J.L.; Bennett, G.A.; Côté, L.M.; Buck, W.B. Comparative Pathologic Changes in Broiler Chicks on Feed Amended with Fusarium Proliferatum Culture Material or Purified Fumonisin B1 and Moniliformin. Mycopathologia 2005, 159, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.T.; Auvergne, A.; Benard, G.; Bailly, J.D.; Tardieu, D.; Babilé, R.; Guerre, P. Chronic Effects of Fumonisin B1 on Ducks. Poult. Sci. 2005, 84, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Hu, H.; Liu, X.; Wang, Y.; Saleemi, M.K.; He, C.; Haque, M.A. Protocatechuic Acid: A Novel Detoxication Agent of Fumonisin B1 for Poultry Industry. Front. Vet. Sci. 2022, 9, 923238. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Brunner, B.; Bielohuby, M.; Hansen, G.; Pfenninger, A.; Wang, B.; Bruning, J.C.; Larsen, P.J.; Tennagels, N. The Role of C16:0 Ceramide in the Development of Obesity and Type 2 Diabetes: CerS6 Inhibition as a Novel Therapeutic Approach. Mol. Metab. 2019, 21, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Turpin-Nolan, S.M.; Hammerschmidt, P.; Chen, W.; Jais, A.; Timper, K.; Awazawa, M.; Brodesser, S.; Brüning, J.C. CerS1-Derived C18:0 Ceramide in Skeletal Muscle Promotes Obesity-Induced Insulin Resistance. Cell Rep. 2019, 26, 1–10.e7. [Google Scholar] [CrossRef] [Green Version]
- Tosetti, B.; Brodesser, S.; Brunn, A.; Deckert, M.; Blüher, M.; Doehner, W.; Anker, S.D.; Wenzel, D.; Fleischmann, B.; Pongratz, C.; et al. A Tissue-Specific Screen of Ceramide Expression in Aged Mice Identifies Ceramide Synthase-1 and Ceramide Synthase-5 as Potential Regulators of Fiber Size and Strength in Skeletal Muscle. Aging Cell 2020, 19, e13049. [Google Scholar] [CrossRef] [PubMed]
- Błachnio-Zabielska, A.U.; Roszczyc-Owsiejczuk, K.; Imierska, M.; Pogodzińska, K.; Rogalski, P.; Daniluk, J.; Zabielski, P. CerS1 but Not CerS5 Gene Silencing, Improves Insulin Sensitivity and Glucose Uptake in Skeletal Muscle. Cells 2022, 11, 206. [Google Scholar] [CrossRef]
- Cingolani, F.; Futerman, A.H.; Casas, J. Ceramide Synthases in Biomedical Research. Chem. Phys. Lipids 2016, 197, 25–32. [Google Scholar] [CrossRef]
- Truman, J.-P.; Ruiz, C.F.; Montal, E.; Garcia-Barros, M.; Mileva, I.; Snider, A.J.; Hannun, Y.A.; Obeid, L.M.; Mao, C. 1-Deoxysphinganine Initiates Adaptive Responses to Serine and Glycine Starvation in Cancer Cells via Proteolysis of Sphingosine Kinase. J. Lipid Res. 2022, 63, 100154. [Google Scholar] [CrossRef]
- Laurain, J.; Tardieu, D.; Matard-Mann, M.; Rodriguez, M.A.; Guerre, P. Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins 2021, 13, 701. [Google Scholar] [CrossRef]
- Sousa, M.C.S.; Galli, G.M.; Alba, D.F.; Griss, L.G.; Gebert, R.R.; Souza, C.F.; Baldissera, M.D.; Gloria, E.M.; Mendes, R.E.; Zanelato, G.O.; et al. Pathogenetic Effects of Feed Intake Containing of Fumonisin (Fusarium Verticillioides) in Early Broiler Chicks and Consequences on Weight Gain. Microb. Pathog. 2020, 147, 104247. [Google Scholar] [CrossRef] [PubMed]
- Bergouignan, A.; Trudel, G.; Simon, C.; Chopard, A.; Schoeller, D.A.; Momken, I.; Votruba, S.B.; Desage, M.; Burdge, G.C.; Gauquelin-Koch, G.; et al. Physical Inactivity Differentially Alters Dietary Oleate and Palmitate Trafficking. Diabetes 2009, 58, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, R.J.; Norris, M.K.; Poss, A.M.; Holland, W.L.; Summers, S.A. The Lard Works in Mysterious Ways: Ceramides in Nutrition-Linked Chronic Disease. Annu. Rev. Nutr. 2022, 42, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Mycotoxin and Gut Microbiota Interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Beresewicz, A.; Dobrzyń, A.; Górski, J. Accumulation of Specific Ceramides in Ischemic/Reperfused Rat Heart; Effect of Ischemic Preconditioning. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2002, 53, 371–382. [Google Scholar]
- Reforgiato, M.R.; Milano, G.; Fabriàs, G.; Casas, J.; Gasco, P.; Paroni, R.; Samaja, M.; Ghidoni, R.; Caretti, A.; Signorelli, P. Inhibition of Ceramide de Novo Synthesis as a Postischemic Strategy to Reduce Myocardial Reperfusion Injury. Basic Res. Cardiol. 2016, 111, 12. [Google Scholar] [CrossRef]
- Egom, E.E.; Mamas, M.A.; Chacko, S.; Stringer, S.E.; Charlton-Menys, V.; El-Omar, M.; Chirico, D.; Clarke, B.; Neyses, L.; Cruickshank, J.K.; et al. Serum Sphingolipids Level as a Novel Potential Marker for Early Detection of Human Myocardial Ischaemic Injury. Front. Physiol. 2013, 4, 130. [Google Scholar] [CrossRef] [Green Version]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic Very-Long-Chain Ceramides Cause Mitochondrial Dysfunction, Oxidative Stress, and Cell Death in Cardiomyocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 1403–1416. [Google Scholar] [CrossRef]
- ANSES_GuideValidation.Pdf. Available online: https://www.anses.fr/fr/system/files/ANSES_GuideValidation.pdf (accessed on 19 December 2018).





| Heart | Gizzard | Breast Muscle | ||||
|---|---|---|---|---|---|---|
| Control | FB | Control | FB | Control | FB | |
| Sphingoid bases and derivates | ||||||
| d18:1 (So) | 5326 ± 596 | 5891 ± 1086 | 1367 ± 387 | 1157 ± 183 | 2585 ± 636 | 3107 ± 388 * |
| d18:0 (Sa) | 868 ± 133 | 1204 ± 222 *** | 233 ± 19 | 213 ± 24 * | 127 ± 89 | 110 ± 72 |
| 18:1/2:0 | 62 ± 13 | 69 ± 11 | 27 ± 8 | 29 ± 9 | 24 ± 8 | 23 ± 7 |
| 18:0/2:0 | ND | ND | 16 ± 5 | 25 ± 7 ** | ND | ND |
| LysoSM | 249 ± 41 | 234 ± 33 | 353 ± 34 | 327 ± 47 | 130 ± 29 | 122 ± 23 |
| Ceramides and dehydroceramides | ||||||
| 18:1/14:0 | 5277 ± 894 | 6217 ± 1588 | 307 ± 85 | 204 ± 45 ** | 75 ± 22 | 70 ± 9 |
| 18:1/16:0 | 77,884 ± 12,241 | 90,785 ± 21,079 | 110,479 ± 33,863 | 81,436 ± 18,040 * | 14,530 ± 5548 | 13,523 ± 2337 |
| 18:0/16:0 | 1125 ± 193 | 1353 ± 298 | 1753 ± 533 | 1089 ± 104 ** | 375 ± 79 | 355 ± 40 |
| 18:1/18:1 | 1036 ± 225 | 1232 ± 243 | 343 ± 132 | 329 ± 102 | ND | ND |
| 18:1/18:0 | 25,264 ± 4163 | 30,555 ± 10,317 | 22,600 ± 8108 | 17,194 ± 4879 | 15,646 ± 4753 | 15,281 ± 3584 |
| 18:0/18:0 | 62 ± 20 | 82 ± 35 | 328 ± 81 | 225 ± 44 ** | 33 ± 12 | 40 ± 13 |
| 18:1/20:0 | 17,052 ± 2930 | 22,897 ± 5917 * | 17,282 ± 5215 | 13,521 ± 3411 | 3574 ± 1426 | 3510 ± 889 |
| 18:1/22:2 | 1556 ± 344 | 1882 ± 549 | 49,559 ± 14,820 | 36,320 ± 11,872 * | 308 ± 97 | 290 ± 67 |
| 18:1/22:1 | 681 ± 130 | 972 ± 292 * | 12,002 ± 5214 | 8371 ± 2826 | 314 ± 103 | 299 ± 59 |
| 18:1/22:0 | 31,576 ± 4466 | 39,187 ± 8219 * | 26,966 ± 7331 | 21,517 ± 4277 | 6466 ± 2186 | 6222 ± 1146 |
| 18:0/22:0 | 60 ± 19 | 76 ± 33 | 223 ± 53 | 168 ± 46 ** | ND | ND |
| 18:1/23:1 | ND | ND | 991 ± 344 | 772 ± 215 | ND | ND |
| 18:1/23:0 | 2140 ± 341 | 2553 ± 496 * | 7308 ± 1401 | 6070 ± 988 * | 445 ± 128 | 402 ± 80 |
| 18:0/23:0 | 23 ± 9 | 31 ± 12 | ND | ND | ND | ND |
| 18:1/24:2 | 3273 ± 714 | 4170 ± 1002 * | 272,361 ± 78,578 | 177,125 ± 43,980 ** | 1380 ± 435 | 1377 ± 292 |
| 18:1/24:1 | 25,974 ± 4601 | 32,914 ± 9442 | 71,565 ± 28,535 | 54,187 ± 16,562 | 11,736 ± 3967 | 11,664 ± 1939 |
| 18:1/24:0 | 20,773 ± 2780 | 23,963 ± 4458 | 14,143 ± 2922 | 12,127 ± 1801 | 4563 ± 1261 | 4399 ± 1009 |
| 18:0/24:0 | 683 ± 139 | 852 ± 349 | ND | ND | ND | ND |
| 18:1/26:2 | 539 ± 125 | 713 ± 197 * | 2815 ± 985 | 1862 ± 383 * | 26 ± 20 | 21 ± 8 |
| 18:1/26:1 | 444 ± 91 | 526 ± 135 | 1270 ± 333 | 881 ± 243 * | 32 ± 16 | 33 ± 17 |
| 18:1/26:0 | ND | ND | 230 ± 82 | 151 ± 38 * | ND | ND |
| Sphingomyelins and dehydrosphingomyelins | ||||||
| SM18:1/14:0 | 13,516 ± 2450 | 12,256 ± 1114 | 1962 ± 424 | 1749 ± 274 | 1024 ± 250 | 1054 ± 150 |
| SM18:1/16:0 | 279,024 ± 24,417 | 269,938 ± 20,805 | 296,493 ± 37,427 | 283,011 ± 26,615 | 142,118 ± 28,443 | 134,059 ± 11,388 |
| SM18:0/16:0 | 29,224 ± 3338 | 30,528 ± 3387 | 287,685 ± 53,159 | 268,997 ± 52,074 | 14,195 ± 4236 | 14,297 ± 2589 |
| SM18:1/18:1 | 7638 ± 1747 | 8262 ± 1449 | ND | ND | ND | ND |
| SM18:1/18:0 | 718,620 ± 70,463 | 774,687 ± 75,203 | 455,089 ± 63,676 | 452,100 ± 59,844 | 557,238 ± 118,116 | 576,469 ± 66,525 |
| SM18:0/18:0 | 9964 ± 2069 | 10,078 ± 2031 | 20,824 ± 4824 | 19,992 ± 6472 | 2876 ± 682 | 2993 ± 579 |
| SM18:1/20:0 | 131,232 ± 8241 | 147,199 ± 14,149 ** | 46,568 ± 5174 | 46,010 ± 6491 | 33,633 ± 10,149 | 34,817 ± 5905 |
| SM18:0/20:0 | 1780 ± 228 | 1898 ± 404 | 2872 ± 646 | 2899 ± 819 | 413 ± 94 | 414 ± 131 |
| SM18:1/22:2 | 7332 ± 1102 | 8022 ± 786 | 70,886 ± 24,225 | 61,750 ± 14,671 | 2554 ± 559 | 2480 ± 283 |
| SM18:1/22:1 | 8259 ± 747 | 9199 ± 1216 | 32,164 ± 5132 | 31,112 ± 8408 | 4092 ± 1083 | 3931 ± 687 |
| SM18:1/22:0 | 509,447 ± 41,889 | 546,720 ± 65,977 | 221,170 ± 32,543 | 201,609 ± 30,819 | 69,740 ± 18,958 | 69,604 ± 15,227 |
| SM18:0/22:0 | 5074 ± 925 | 5230 ± 1059 | 3921 ± 1054 | 3492 ± 1033 | 520 ± 140 | 498 ± 102 |
| SM18:1/23:1 | 7885 ± 1113 | 8422 ± 1406 | 5465 ± 1250 | 4849 ± 958 | 2787 ± 927 | 2969 ± 955 |
| SM18:1/23:0 | 27,322 ± 3979 | 29,310 ± 5566 | 33,713 ± 7600 | 34,101 ± 12,246 | 2134 ± 578 | 2062 ± 468 |
| SM18:0/23:0 | 3984 ± 755 | 4392 ± 1105 | 466 ± 90 | 423 ± 115 | 765 ± 269 | 705 ± 180 |
| SM18:1/24:3 | ND | ND | 19 ± 2 | 18 ± 1 | 23 ± 1 | 24 ± 2 |
| SM18:1/24:2 | 30,205 ± 4115 | 31,127 ± 3310 | 1078,170 ± 385,433 | 876,991 ± 143,010 | 14,609 ± 4097 | 14,083 ± 2864 |
| SM18:1/24:1 | 254,344 ± 30,783 | 271,904 ± 47,893 | 381,839 ± 65,468 | 378,196 ± 73,027 | 57,450 ± 14,974 | 58,443 ± 14,194 |
| SM18:1/24:0 | 152,411 ± 18,191 | 166,908 ± 26,357 | 76,543 ± 12,027 | 70,781 ± 14,501 | 15,543 ± 4195 | 15,449 ± 3559 |
| SM18:0/24:1 | 2453 ± 434 | 2665 ± 483 | 6900 ± 2267 | 6308 ± 2712 | 319 ± 75 | 333 ± 89 |
| SM18:0/24:0 | 605 ± 104 | 564 ± 124 | 954 ± 281 | 794 ± 179 | ND | ND |
| SM18:1/25:2 | 3295 ± 2038 | 3682 ± 2300 | 7145 ± 1906 | 6169 ± 1291 | 239 ± 60 | 211 ± 38 |
| SM18:1/25:1 | 1527 ± 243 | 1771 ± 348 | 5960 ± 1313 | 5792 ± 1904 | 303 ± 77 | 285 ± 93 |
| SM18:1/25:0 | 817 ± 215 | 948 ± 221 | 1493 ± 422 | 1478 ± 453 | 108 ± 26 | 106 ± 28 |
| SM18:1/26:3 | 5393 ± 677 | 5784 ± 586 | 6079 ± 2098 | 5165 ± 1483 | 324 ± 119 | 381 ± 123 |
| SM18:1/26:2 | 8088 ± 1363 | 9027 ± 1297 | 35,708 ± 11,840 | 30,033 ± 8733 | 529 ± 182 | 565 ± 217 |
| SM18:1/26:1 | 2500 ± 483 | 2742 ± 485 | 12,702 ± 3216 | 11,639 ± 3881 | 307 ± 101 | 329 ± 97 |
| SM18:1/26:0 | ND | ND | 857 ± 245 | 841 ± 291 | ND | ND |
| Hexosyl-, and lactosylceramides, and ceramides sulfatides | ||||||
| Hex18:1/16:0 | 2695 ± 477 | 2789 ± 640 | 730 ± 250 | 601 ± 143 | 832 ± 257 | 781 ± 203 |
| Hex18:1/18:0 | 20,024 ± 4349 | 23,297 ± 5049 | 2913 ± 1341 | 2329 ± 1234 | ND | ND |
| Hex18:1/20:0 | 3123 ± 538 | 3534 ± 1098 | 676 ± 283 | 688 ± 419 | ND | ND |
| Hex18:1/22:0 | 2199 ± 370 | 2811 ± 752 * | 1179 ± 716 | 874 ± 385 | ND | ND |
| Hex18:1/24:1 | 14,817 ± 1554 | 17,483 ± 3396 * | 1160 ± 660 | 637 ± 261 * | 1340 ± 976 | 976 ± 518 |
| Hex18:1/24:0 | 4285 ± 875 | 5119 ± 1405 | 934 ± 351 | 905 ± 466 | ND | ND |
| Lac18:1/16:0 | 3280 ± 663 | 3293 ± 1192 | 5131 ± 1257 | 3919 ± 1036 * | 1172 ± 441 | 1091 ± 162 |
| Lac18:1/18:0 | 164,969 ± 26,340 | 195,360 ± 49,026 | 24,678 ± 6030 | 23,262 ± 8744 | 16,877 ± 6682 | 18,010 ± 4202 |
| Lac18:1/20:0 | 16,080 ± 3467 | 20,537 ± 7002 | 971 ± 667 | 764 ± 424 | 7237 ± 3232 | 7812 ± 2430 |
| Lac18:1/22:0 | 11,757 ± 4980 | 17,071 ± 10,225 | 2325 ± 821 | 2803 ± 1798 | 1432 ± 654 | 1783 ± 610 |
| Lac18:1/24:1 | 17,053 ± 2330 | 22,119 ± 5235 * | 3281 ± 970 | 3747 ± 2019 | 18,040 ± 8822 | 23,515 ± 7061 |
| Lac18:1/24:0 | 18,598 ± 3893 | 24,212 ± 8371 | ND | ND | 2308 ± 1149 | 2441 ± 785 |
| ST18:1/24:1 | 39,266 ± 6545 | 46,422 ± 11,226 | ND | ND | ND | ND |
| ST18:1/24:0 | 56,643 ± 9617 | 71,979 ± 13,510 ** | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerre, P.; Gilleron, C.; Matard-Mann, M.; Nyvall Collén, P. Targeted Sphingolipid Analysis in Heart, Gizzard, and Breast Muscle in Chickens Reveals Possible New Target Organs of Fumonisins. Toxins 2022, 14, 828. https://doi.org/10.3390/toxins14120828
Guerre P, Gilleron C, Matard-Mann M, Nyvall Collén P. Targeted Sphingolipid Analysis in Heart, Gizzard, and Breast Muscle in Chickens Reveals Possible New Target Organs of Fumonisins. Toxins. 2022; 14(12):828. https://doi.org/10.3390/toxins14120828
Chicago/Turabian StyleGuerre, Philippe, Caroline Gilleron, Maria Matard-Mann, and Pi Nyvall Collén. 2022. "Targeted Sphingolipid Analysis in Heart, Gizzard, and Breast Muscle in Chickens Reveals Possible New Target Organs of Fumonisins" Toxins 14, no. 12: 828. https://doi.org/10.3390/toxins14120828
APA StyleGuerre, P., Gilleron, C., Matard-Mann, M., & Nyvall Collén, P. (2022). Targeted Sphingolipid Analysis in Heart, Gizzard, and Breast Muscle in Chickens Reveals Possible New Target Organs of Fumonisins. Toxins, 14(12), 828. https://doi.org/10.3390/toxins14120828