Exploration of Mycotoxin Accumulation and Transcriptomes of Different Wheat Cultivars during Fusarium graminearum Infection
Abstract
:1. Introduction
2. Results
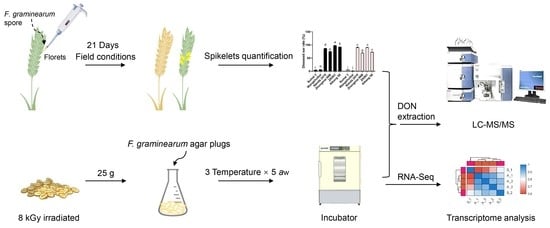
2.1. Evaluation of Toxin Accumulation of Six Wheat Cultivars under Field Conditions
2.2. Accumulation of DON and D3G in F. graminearum PH−1-Infected Wheat Grains under Different aw and Temperature Conditions
2.3. Accumulation of DON and D3G in F. graminearum F1-Infected Wheat Grains under Different aw and Temperature Conditions
2.4. Overview of Differentially Expressed Genes (DEG) between F. graminearum F1-Infected Sumai 3 and Annong 0711 Wheat Grains
2.5. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses of DEGs in Wheat
3. Discussion
4. Materials and Methods
4.1. Wheat Sample and F. graminearum Strains
4.2. Chemicals and Reagents
4.3. Inoculation and Incubation Conditions
4.4. Mycotoxin Extraction
4.5. Mycotoxin Determination by LC–MS/MS
4.6. Total RNA Extraction
4.7. Library Preparation and Sequencing
4.8. Read Mapping and DEG Analysis
4.9. Functional Annotation and Enrichment
4.10. Quantitative Real-Time PCR Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FHB | Fusarium head blight |
| DON | deoxynivalenol |
| D3G | deoxynivalenol-3-glucoside |
| HPLC | high performance liquid chromatography |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| aw | water activity |
| DEG | differentially expressed gene |
| COG | Clusters Orthologous Groups |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genome |
| BP | biological process |
| CC | cellular component |
| MF | molecular function |
| MAPK | mitogen activated protein kinases |
| HR | hypersensitivity response |
References
- Scala, V.; Pietricola, C.; Farina, V.; Beccaccioli, M.; Zjalic, S.; Quaranta, F.; Fornara, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; et al. Tramesan Elicits Durum Wheat Defense against the Septoria Disease Complex. Biomolecules 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiani, M.; Bryan, B.; Rush, C.; Szczepaniec, A. Transcriptional Responses of Resistant and Susceptible Wheat Exposed to Wheat Curl Mite. Int. J. Mol. Sci. 2021, 22, 2703. [Google Scholar] [CrossRef]
- Kikot, G.E.; Moschini, R.; Consolo, V.F.; Rojo, R.; Salerno, G.; Hours, R.A.; Gasoni, L.; Arambarri, A.M.; Alconada, T.M. Occurrence of Different Species of Fusarium from Wheat in Relation to Disease Levels Predicted by a Weather-Based Model in Argentina Pampas Region. Mycopathologia 2011, 171, 139–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, X.; Zong, Y.; Hua, X.; Xing, F.; Wang, Y.; Wang, F.; Liu, Y. Deoxynivalenol in wheat from the Northwestern region in China. Food Addit. Contam. Part B 2018, 11, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabre, F.; Rocher, F.; Alouane, T.; Langin, T.; Bonhomme, L. Searching for FHB Resistances in Bread Wheat: Susceptibility at the Crossroad. Front. Plant Sci. 2020, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, G.; Shi, J.; Tian, S.; Zhang, X.; Cheng, R.; Wang, X.; Yuan, Y.; Cao, S.; Zhou, J.; et al. Genetic control of Fusarium head blight resistance in two Yangmai 158-derived recombinant inbred line populations. Theor. Appl. Genet. 2021, 134, 3037–3049. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Ma, H.; Huang, L.; Ding, F.; Du, Y.; Jia, H.; Li, G.; Kong, Z.; Ran, C.; et al. Pyramiding of Fusarium Head Blight Resistance Quantitative Trait Loci, Fhb1, Fhb4, and Fhb5, in Modern Chinese Wheat Cultivars. Front. Plant Sci. 2021, 12, 694023. [Google Scholar] [CrossRef]
- Jia, L.-J.; Tang, H.-Y.; Wang, W.-Q.; Yuan, T.-L.; Wei, W.-Q.; Pang, B.; Gong, X.-M.; Wang, S.-F.; Li, Y.-J.; Zhang, D.; et al. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. Nat. Commun. 2019, 10, 922. [Google Scholar] [CrossRef] [Green Version]
- Chiotta, M.L.; Alaniz Zanon, M.S.; Palazzini, J.M.; Alberione, E.; Barros, G.G.; Chulze, S.N. Fusarium graminearum species complex occurrence on soybean and F. graminearum sensu stricto inoculum maintenance on residues in soybean-wheat rotation under field conditions. J. Appl. Microbiol. 2021, 130, 208–216. [Google Scholar] [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural Occurrence of Deoxynivalenol and Its Acetylated Derivatives in Chinese Maize and Wheat Collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [Green Version]
- Crippin, T.; Renaud, J.B.; Sumarah, M.W.; Miller, J.D. Comparing genotype and chemotype of Fusarium graminearum from cereals in Ontario, Canada. PLoS ONE 2019, 14, e0216735. [Google Scholar] [CrossRef]
- Yu, S.; Jia, B.; Li, K.; Zhou, H.; Lai, W.; Tang, Y.; Yan, Z.; Sun, W.; Liu, N.; Yu, D.; et al. Pre-warning of abiotic factors in maize required for potential contamination of fusarium mycotoxins via response surface analysis. Food Control 2021, 121, 107570. [Google Scholar] [CrossRef]
- Hooft, J.M.; Bureau, D.P. Deoxynivalenol: Mechanisms of action and its effects on various terrestrial and aquatic species. Food Chem. Toxicol. 2021, 157, 112616. [Google Scholar] [CrossRef]
- Guo, H.; Ji, J.; Wang, J.; Sun, X. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirlini, M.; Generotti, S.; Dall’Erta, A.; Lancioni, P.; Ferrazzano, G.; Massi, A.; Galaverna, G.; Dall’Asta, C. Durum Wheat (Triticum Durum Desf.) Lines Show Different Abilities to Form Masked Mycotoxins under Greenhouse Conditions. Toxins 2013, 6, 81–95. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Ahmad, D.; Zhang, X.; Zhang, Y.; Wu, L.; Jiang, P.; Ma, H. Genome-wide analysis of family-1 UDP glycosyltransferases (UGT) and identification of UGT genes for FHB resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2018, 18, 67. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Liu, X.; Jiang, P.; Yu, L.; Qiu, J.; Wang, G.; Zhang, X.; Ma, H. TaUGT6, a Novel UDP-Glycosyltransferase Gene Enhances the Resistance to FHB and DON Accumulation in Wheat. Front. Plant Sci. 2020, 11, 574775. [Google Scholar] [CrossRef]
- Sharma, P.; Gangola, M.P.; Huang, C.; Kutcher, H.R.; Ganeshan, S.; Chibbar, R.N. Single Nucleotide Polymorphisms in B-Genome Specific UDP-Glucosyl Transferases Associated with Fusarium Head Blight Resistance and Reduced Deoxynivalenol Accumulation in Wheat Grain. Phytopathology 2018, 108, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the Mycotoxin Deoxynivalenol in Fusarium Resistant and Susceptible Near Isogenic Wheat Lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during wheat milling and Chinese steamed bread processing. Food Control 2014, 44, 86–91. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachova, A.; et al. In vivo contribution of deoxynivalenol-3-β-D-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017, 91, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Mengelers, M.; Zeilmaker, M.; Vidal, A.; De Boevre, M.; De Saeger, S.; Hoogenveen, R. Biomonitoring of Deoxynivalenol and Deoxynivalenol-3-glucoside in Human Volunteers: Renal Excretion Profiles. Toxins 2019, 11, 466. [Google Scholar] [CrossRef] [Green Version]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The Human Fecal Microbiota Metabolizes Deoxynivalenol and Deoxynivalenol-3-Glucoside and May Be Responsible for Urinary Deepoxy-Deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef] [Green Version]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine Small and Large Intestinal Microbiota Rapidly Hydrolyze the Masked Mycotoxin Deoxynivalenol-3-Glucoside and Release Deoxynivalenol in Spiked Batch Cultures In Vitro. Appl. Environ. Microbiol. 2018, 84, e02106-17. [Google Scholar] [CrossRef] [Green Version]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.-D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.-D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wang, L.; Yu, D.; Yan, Z.; Liu, N.; Wu, A. Cellobiose inhibits the release of deoxynivalenol from transformed deoxynivalenol-3-glucoside from Lactiplantibacillus plantarum. Food Chem. Mol. Sci. 2022, 4, 100077. [Google Scholar] [CrossRef] [PubMed]
- Verheecke-Vaessen, C.; Garcia-Cela, E.; Lopez-Prieto, A.; Jonsdottir, I.O.; Medina, A.; Magan, N. Water and temperature relations of Fusarium langsethiae strains and modelling of growth and T-2 and HT-2 mycotoxin production on oat-based matrices. Int. J. Food Microbiol. 2021, 348, 109203. [Google Scholar] [CrossRef] [PubMed]
- Rybecky, A.; Chulze, S.; Chiotta, M. Effect of water activity and temperature on growth and trichothecene production by Fusarium meridionale. Int. J. Food Microbiol. 2018, 285, 69–73. [Google Scholar] [CrossRef]
- Peter Mshelia, L.; Selamat, J.; Iskandar Putra Samsudin, N.; Rafii, M.Y.; Abdul Mutalib, N.A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Schiro, G.; Müller, T.; Verch, G.; Sommerfeld, T.; Mauch, T.; Koch, M.; Grimm, V.; Müller, M.E. The distribution of mycotoxins in a heterogeneous wheat field in relation to microclimate, fungal and bacterial abundance. J. Appl. Microbiol. 2018, 126, 177–190. [Google Scholar] [CrossRef]
- Fan, P.; Gu, K.; Wu, J.; Zhou, M.; Chen, C. Effect of wheat (Triticum aestivum L.) resistance, Fusarium graminearum DNA content, strain potential toxin production, and disease severity on deoxynivalenol content. J. Basic Microbiol. 2019, 59, 1105–1111. [Google Scholar] [CrossRef]
- Belizán, M.M.; Gomez, A.D.L.A.; Baptista, Z.P.T.; Jimenez, C.M.; Matías, M.D.H.S.; Catalán, C.A.; Sampietro, D.A. Influence of water activity and temperature on growth and production of trichothecenes by Fusarium graminearum sensu stricto and related species in maize grains. Int. J. Food Microbiol. 2019, 305, 108242. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, 822. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Transcriptomics of cereal-Fusarium graminearum interactions: What we have learned so far. Mol. Plant Pathol. 2018, 19, 764–778. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, H.; Yang, J.; Yang, X.; Zhang, M.; Zhao, Z.; Fan, Y.; Wang, C.; Wang, J. Bioinformatics and Transcriptome Analysis of CFEM Proteins in Fusarium graminearum. J. Fungi 2021, 7, 871. [Google Scholar] [CrossRef]
- Buhrow, L.M.; Liu, Z.; Cram, D.; Sharma, T.; Foroud, N.A.; Pan, Y.; Loewen, M.C. Wheat transcriptome profiling reveals abscisic and gibberellic acid treatments regulate early-stage phytohormone defense signaling, cell wall fortification, and metabolic switches following Fusarium graminearum-challenge. BMC Genom. 2021, 22, 798. [Google Scholar] [CrossRef] [PubMed]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium Head Blight in Durum Wheat: Recent Status, Breeding Directions, and Future Research Prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S.; Kistler, H.C. Pathogenicity and In Planta Mycotoxin Accumulation among Members of the Fusarium graminearum Species Complex on Wheat and Rice. Phytopathology 2005, 95, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, P.; Dill-Macky, R. Impact of moisture, host genetics and Fusarium graminearum isolates on Fusarium head blight development and trichothecene accumulation in spring wheat. Mycotoxin Res. 2011, 28, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; De Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wang, T.; Zhu, M.; Zhang, L.; Zhang, F.; Jing, E.; Ren, Y.; Wang, Z.; Xin, Z.; Lin, T. Transcriptome and physiological analyses for revealing genes involved in wheat response to endoplasmic reticulum stress. BMC Plant Biol. 2019, 19, 193. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldbauer, R.; Gosch, L.; Lüftinger, L.; Hyden, P.; Flexer, A.; Rattei, T. DeepNOG: Fast and accurate protein orthologous group assignment. Bioinformatics 2020, 36, 5304–5312. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Shen, S.; Kong, J.; Qiu, Y.; Yang, X.; Wang, W.; Yan, L. Identification of core genes and outcomes in hepatocellular carcinoma by bioinformatics analysis. J. Cell. Biochem. 2019, 120, 10069–10081. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]






| Strain | Wheat Cultivar | FHB Resistance | Conditions | Subject |
|---|---|---|---|---|
| F. graminearum PH−1 F. graminearum F1 | Sumai 3 | Resistant | Field conditions | Wheat spikes * [9] |
| Wangshuibai | Resistant | |||
| ZK001 | Moderately Resistant | |||
| Nanda 2419 | Moderately Resistant | |||
| Aikang 58 | Susceptible | |||
| Zhongmai 66B | Susceptible | |||
| F. graminearum PH−1 F. graminearum F1 | Sumai 3 | Resistant | aw: 0.80, 0.85, 0.9, 0.95, 0.99 T (°C): 20, 25, 30 [15] | Post-harvest wheat grains |
| Annong 0711 | Moderately Resistant | |||
| Zhongmai 66B | Susceptible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Yu, D.; Yan, Z.; Liu, N.; Fan, Y.; Wang, C.; Wu, A. Exploration of Mycotoxin Accumulation and Transcriptomes of Different Wheat Cultivars during Fusarium graminearum Infection. Toxins 2022, 14, 482. https://doi.org/10.3390/toxins14070482
Li K, Yu D, Yan Z, Liu N, Fan Y, Wang C, Wu A. Exploration of Mycotoxin Accumulation and Transcriptomes of Different Wheat Cultivars during Fusarium graminearum Infection. Toxins. 2022; 14(7):482. https://doi.org/10.3390/toxins14070482
Chicago/Turabian StyleLi, Kailin, Dianzhen Yu, Zheng Yan, Na Liu, Yingying Fan, Cheng Wang, and Aibo Wu. 2022. "Exploration of Mycotoxin Accumulation and Transcriptomes of Different Wheat Cultivars during Fusarium graminearum Infection" Toxins 14, no. 7: 482. https://doi.org/10.3390/toxins14070482
APA StyleLi, K., Yu, D., Yan, Z., Liu, N., Fan, Y., Wang, C., & Wu, A. (2022). Exploration of Mycotoxin Accumulation and Transcriptomes of Different Wheat Cultivars during Fusarium graminearum Infection. Toxins, 14(7), 482. https://doi.org/10.3390/toxins14070482