Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function
Abstract
:1. Introduction
2. Results
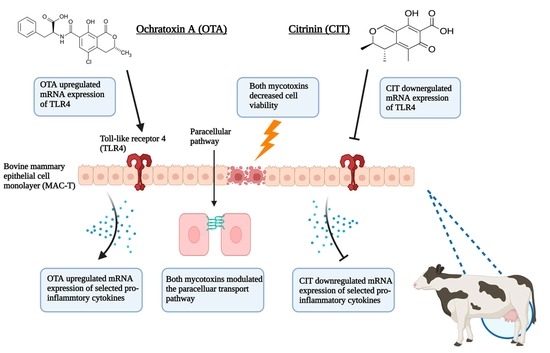
2.1. Cytotoxic Effects of OTA and CIT
2.2. Effects of OTA and CIT on Paracellular Permeability
2.3. Gene Expression Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Cell Culture
5.3. Cytotoxicity Assay
5.4. Transepithelial Electrical Resistance (TEER) Measurement
5.5. Permeability Tracer Flux Assay
5.6. Quantitative Real-Time PCR Analysis
5.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, D.K.; Kang, J.I.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Simal-Gandara, J.; Xiao, J.; Ali, S.; Huh, Y.S.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. The Status of Fusarium Mycotoxins in Sub-Saharan Africa: A Review of Emerging Trends and Post-Harvest Mitigation Strategies towards Food Control. Toxins 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Kępińska-Pacelik, J.; Biel, W. Mycotoxins—Prevention, Detection, Impact on Animal Health. Processes 2021, 9, 2035. [Google Scholar] [CrossRef]
- Weaver, A.C.; King, W.D.; Verax, M.; Fox, U.; Kudupoje, M.B.; Mathis, G.; Lumpkins, B.; Yiannikouris, A. Impact of Chronic Levels of Naturally Multi-Contaminated Feed with Fusarium Mycotoxins on Broiler Chickens and Evaluation of the Mitigation Properties of Different Titers of Yeast Cell Wall Extract. Toxins 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Priest, E.; Naglik, J.R.; Richardson, J.P. Fungal Toxins and Host Immune Responses. Front. Microbiol. 2021, 12, 643639. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.M.; Oh, S.-Y.; Yiannikouris, A.; Graugnard, D.E.; Karrow, N.A. Differential Gene Expression Analysis of Bovine Macrophages after Exposure to the Penicillium Mycotoxins Citrinin and/or Ochratoxin A. Toxins 2017, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- EFSA; Maggiore, A.; Afonso, A.; Barrucci, F.; De Sanctis, G. Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar] [CrossRef]
- FAO. Climate Change: Unpacking the Burden on Food Safety; Food Safety and Quality Series; FAO: Rome, Italy, 2020; ISBN 978-92-5-132293-2. [Google Scholar]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the Impact of Mycotoxins on Dairy Cattle Health: Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef]
- Akinmusire, O.O.; El-Yuguda, A.-D.; Musa, J.A.; Oyedele, O.A.; Sulyok, M.; Somorin, Y.M.; Ezekiel, C.N.; Krska, R. Mycotoxins in poultry feed and feed ingredients in Nigeria. Mycotoxin Res. 2018, 35, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Weaver, A.; Weaver, D.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.; Khatoon, A.; Arooj, N.; Hussain, S.; Ali, S.; Manzoor, A.W.; Saleemi, M.K. Estimation of ochratoxin A in poultry feed and its ingredients with special reference to temperature conditions. Br. Poult. Sci. 2017, 58, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Gumus, R.; Ercan, N.; Imik, H. Determination of Ochratoxin A Levels in Mixed Feed and Feed Stuffs Used in Some Laying Hens and Ruminant Enterprises of Sivas City. Braz. J. Poult. Sci. 2018, 20, 85–90. [Google Scholar] [CrossRef]
- Leiva, A.; Méndez, G.; Rodríguez, C.; Molina, A.; Granados-Chinchilla, F. Chemical assessment of mycotoxin contaminants and veterinary residues in Costa Rican animal feed. Int. J. Food Contam. 2019, 6, 5. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (Contam). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; di Mavungu, J.D.; Huybrechts, B.; Tangni, E.K.; Devreese, M.; Croubels, S.; De Saeger, S. Development and validation of an LC–MS/MS method for the simultaneous determination of citrinin and ochratoxin a in a variety of feed and foodstuffs. J. Chromatogr. A 2018, 1580, 100–109. [Google Scholar] [CrossRef]
- European Food Safety. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004, 2, 101. [Google Scholar] [CrossRef]
- Kelman, M.; Renaud, J.; Baines, D.; Yeung, K.-C.; Miller, J.; Sumarah, M. Mycotoxin determination in fungal contaminated Canadian silage toxic to dairy cows and goats. World Mycotoxin J. 2022, 15, 429–438. [Google Scholar] [CrossRef]
- Billenkamp, F.; Schnabel, K.; Hüther, L.; Frahm, J.; von Soosten, D.; Meyer, U.; Höper, D.; Beer, M.; Seyboldt, C.; Neubauer, H.; et al. No hints at glyphosate-induced ruminal dysbiosis in cows. npj Biofilms Microbiomes 2021, 7, 30. [Google Scholar] [CrossRef]
- Huang, Y.; Marden, J.P.; Julien, C.; Bayourthe, C. Redox potential: An intrinsic parameter of the rumen environment. J. Anim. Physiol. Anim. Nutr. 2018, 102, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Ozbey, F. Assessment of the bioaccessibility of aflatoxins from various food matrices using an in vitro digestion model, and the efficacy of probiotic bacteria in reducing bioaccessibility. J. Food Compos. Anal. 2012, 27, 21–31. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne Mycotoxins Beauvericin and Enniatins and Livestock Animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pamel, E.; Daeseleire, E.; De Boevre, M.; De Saeger, S.; Vidal, A.; et al. The role of roughage provision on the absorption and disposition of the mycotoxin deoxynivalenol and its acetylated derivatives in calves: From field observations to toxicokinetics. Arch. Toxicol. 2018, 93, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Li, J.; Yang, Y.; Wang, P.; Yan, F.; Yao, J.; Wu, S. Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis. npj Biofilms Microbiomes 2021, 7, 45. [Google Scholar] [CrossRef]
- Debevere, S.; Cools, A.; De Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, M.A.; Ha, J.K. Mycotoxins and Their Biotransformation in the Rumen: A Review. Asian-Australas. J. Anim. Sci. 2010, 23, 1250–1260. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Becker-Algeri, T.A.; Castagnaro, D.; Bortoli, K.; Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [Green Version]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.-P. The Carry-Over of Mycotoxins in Products of Animal Origin with Special Regard to Its Implications for the European Food Safety Legislation. Food Nutr. Sci. 2011, 2011, 852–867. [Google Scholar] [CrossRef]
- Kessler, E.; Wall, S.; Hernandez, L.; Gross, J.; Bruckmaier, R. Short communication: Mammary gland tight junction permeability after parturition is greater in dairy cows with elevated circulating serotonin concentrations. J. Dairy Sci. 2019, 102, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Bruckmaier, R. Invited review: The role of the blood–milk barrier and its manipulation for the efficacy of the mammary immune response and milk production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. The epithelial barrier and beyond: Claudins as amplifiers of physiological organ functions. IUBMB Life 2017, 69, 290–296. [Google Scholar] [CrossRef]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet.-Res. 2014, 45, 16. [Google Scholar] [CrossRef]
- Gray, C.; Strandberg, Y.; Donaldson, L.; Tellam, R.L. Bovine mammary epithelial cells, initiators of innate immune responses to mastitis. Aust. J. Exp. Agric. 2005, 45, 757–761. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet.-Res. 2006, 37, 369–400. [Google Scholar] [CrossRef]
- Sun, L.; Chen, L.; Wang, F.; Zheng, X.; Yuan, C.; Niu, Q.; Li, Z.; Deng, L.; Zheng, B.; Li, C.; et al. Exogenous hydrogen sulfide prevents lipopolysaccharide-induced inflammation by blocking the TLR4/NF-κB pathway in MAC-T cells. Gene 2019, 710, 114–121. [Google Scholar] [CrossRef]
- Zhuang, C.; Liu, G.; Barkema, H.W.; Zhou, M.; Xu, S.; Rahman, S.U.; Liu, Y.; Kastelic, J.P.; Gao, J.; Han, B. Selenomethionine Suppressed TLR4/NF-κB Pathway by Activating Selenoprotein S to Alleviate ESBL Escherichia coli-Induced Inflammation in Bovine Mammary Epithelial Cells and Macrophages. Front. Microbiol. 2020, 11, 1461. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Varasteh, S.; Braber, S.; Malekinejad, H.; Fink-Gremmels, J. Ochratoxin A challenges the intestinal epithelial cell integrity: Results obtained in model experiments with Caco-2 cells. World Mycotoxin J. 2019, 12, 399–407. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins 2017, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ye, Q.; Bao, X.; Huang, X.; Wang, J.; Zheng, N. Transcriptomic and proteomic profiling reveals the intestinal immunotoxicity induced by aflatoxin M1 and ochratoxin A. Toxicon 2020, 180, 49–61. [Google Scholar] [CrossRef]
- Nakayama, H.; Kitagawa, N.; Otani, T.; Iida, H.; Anan, H.; Inai, T. Ochratoxin A, citrinin and deoxynivalenol decrease claudin-2 expression in mouse rectum CMT93-II cells. Microscopy 2018, 67, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Saif, L.J. Bovine Immunology: Implications for Dairy Cattle. Front. Immunol. 2021, 12, 659801. [Google Scholar] [CrossRef]
- Mitz, C.A.; Viloria-Petit, A.M. TGF-beta signalling in bovine mammary gland involution and a comparative assessment of MAC-T and BME-UV1 cells as in vitro models for its study. PeerJ 2019, 6, e6210. [Google Scholar] [CrossRef]
- Silva, L.G.; Ferguson, B.S.; Faciola, A.P. Rapid Communication: Prolactin and hydrocortisone impact TNFα-mediated mitogen-activated protein kinase signaling and inflammation of bovine mammary epithelial (MAC-T) cells. J. Anim. Sci. 2017, 95, 5524–5531. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Y.; Wu, S.; Yu, H.; Zhao, Y.; Fang, H.; Shen, J.; Zhou, C.; Fu, Y.; Li, R.; et al. Deoxynivalenol induces oxidative stress, inflammatory response and apoptosis in bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, X.; Sun, L.; He, T.; Wei, R.; Pang, M.; Wang, R. Staphylococcus aureus Bacteriophage Suppresses LPS-Induced Inflammation in MAC-T Bovine Mammary Epithelial Cells. Front. Microbiol. 2018, 9, 1614. [Google Scholar] [CrossRef] [Green Version]
- Bertero, A.; Fossati, P.; Tedesco, D.E.A.; Caloni, F. Beauvericin and Enniatins: In Vitro Intestinal Effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Cedergreen, N.; Yiannikouris, A.; Swamy, H.; Karrow, N.A. Assessing interactions of binary mixtures of Penicillium mycotoxins (PMs) by using a bovine macrophage cell line (BoMacs). Toxicol. Appl. Pharmacol. 2017, 318, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewski, P.; Twarużek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Karrow, N.A.; Shandilya, U.K.; Sun, L.-H.; Kitazawa, H. In-Vitro Cell Culture for Efficient Assessment of Mycotoxin Exposure, Toxicity and Risk Mitigation. Toxins 2020, 12, 146. [Google Scholar] [CrossRef]
- Hervé, L.; Quesnel, H.; Lollivier, V.; Boutinaud, M. Regulation of cell number in the mammary gland by controlling the exfoliation process in milk in ruminants. J. Dairy Sci. 2016, 99, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Ling, K.-H.; Wan, M.L.Y.; El-Nezami, H.; Wang, M. Protective Capacity of Resveratrol, a Natural Polyphenolic Compound, against Deoxynivalenol-Induced Intestinal Barrier Dysfunction and Bacterial Translocation. Chem. Res. Toxicol. 2016, 29, 823–833. [Google Scholar] [CrossRef]
- Rainard, P.; Fromageau, A.; Cunha, P.; Gilbert, F.B. Staphylococcus aureuslipoteichoic acid triggers inflammation in the lactating bovine mammary gland. Vet.-Res. 2008, 39, 52. [Google Scholar] [CrossRef]
- Li, Z.; You, Q.; Ossa, F.; Mead, P.; Quinton, M.; Karrow, N.A. Assessment of yeast Saccharomyces cerevisiae component binding to Mycobacterium avium subspecies paratuberculosis using bovine epithelial cells. BMC Vet.-Res. 2016, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Kang, H.; You, Q.; Ossa, F.; Mead, P.; Quinton, M.; Karrow, N. In vitro bioassessment of the immunomodulatory activity of Saccharomyces cerevisiae components using bovine macrophages and Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2018, 101, 6271–6286. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Quinton, V.M.; Boermans, H.J.; Swamy, H.V.L.N.; Karrow, N.A. In vitro exposure of Penicillium mycotoxins with or without a modified yeast cell wall extract (mYCW) on bovine macrophages (BoMacs). Mycotoxin Res. 2015, 31, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V.S.; Alshatwi, A.; Akbarsha, M.A. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015, 83, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhu, H.; Li, T.; Ming, G.; Duan, X.; Wang, J.; Jiang, Y. Molecular signatures of cytotoxic effects in human embryonic kidney 293 cells treated with single and mixture of ochratoxin A and citrinin. Food Chem. Toxicol. 2018, 123, 374–384. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lim, W.; Ryu, S.; Kim, J.; Song, G. Ochratoxin A mediates cytotoxicity through the MAPK signaling pathway and alters intracellular homeostasis in bovine mammary epithelial cells. Environ. Pollut. 2018, 246, 366–373. [Google Scholar] [CrossRef]
- Pinhão, M.; Tavares, A.; Loureiro, S.; Louro, H.; Alvito, P.; Silva, M. Combined cytotoxic and genotoxic effects of ochratoxin A and fumonisin B1 in human kidney and liver cell models. Toxicol. Vitr. 2020, 68, 104949. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhai, N.; Chen, X.; Gan, F.; Li, H.; Huang, K. Ochratoxin A-Induced Apoptosis of IPEC-J2 Cells through ROS-Mediated Mitochondrial Permeability Transition Pore Opening Pathway. J. Agric. Food Chem. 2017, 65, 10630–10637. [Google Scholar] [CrossRef]
- Klarić, M.; Želježić, D.; Rumora, L.; Peraica, M.; Pepeljnjak, S.; Domijan, A.-M. A potential role of calcium in apoptosis and aberrant chromatin forms in porcine kidney PK15 cells induced by individual and combined ochratoxin A and citrinin. Arch. Toxicol. 2011, 86, 97–107. [Google Scholar] [CrossRef]
- Knecht, A.; Schwerdt, G.; Gekle, M.; Humpf, H.-U. Combinatory effects of citrinin and ochratoxin A in immortalized human proximal tubule cells. Mycotoxin Res. 2005, 21, 176–181. [Google Scholar] [CrossRef]
- Montalbetti, N.; Dalghi, M.G.; Albrecht, C.; Hediger, M.A. Nutrient Transport in the Mammary Gland: Calcium, Trace Minerals and Water Soluble Vitamins. J. Mammary Gland Biol. Neoplasia 2014, 19, 73–90. [Google Scholar] [CrossRef]
- Stelwagen, K.; Singh, K. The Role of Tight Junctions in Mammary Gland Function. J. Mammary Gland Biol. Neoplasia 2013, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Alvito, P.; Kleiveland, C.R.; Lea, T.E. Characterization of in vitro effects of patulin on intestinal epithelial and immune cells. Toxicol. Lett. 2016, 250–251, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Pinhão, M.; Loureiro, S.; Alvito, P.; Silva, M.J. A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells. Toxicol. Lett. 2019, 313, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Binder, L.; Pantakani, D.V.K.; Asif, A.R. MPA Modulates Tight Junctions’ Permeability via Midkine/PI3K Pathway in Caco-2 Cells: A Possible Mechanism of Leak-Flux Diarrhea in Organ Transplanted Patients. Front. Physiol. 2017, 8, 438. [Google Scholar] [CrossRef]
- Luo, S.; Terciolo, C.; Bracarense, A.P.L.; Payros, D.; Pinton, P.; Oswald, I.P. In vitro and in vivo effects of a mycotoxin, deoxynivalenol, and a trace metal, cadmium, alone or in a mixture on the intestinal barrier. Environ. Int. 2019, 132, 105082. [Google Scholar] [CrossRef]
- Springler, A.; Vrubel, G.-J.; Mayer, E.; Schatzmayr, G.; Novak, B. Effect of Fusarium-Derived Metabolites on the Barrier Integrity of Differentiated Intestinal Porcine Epithelial Cells (IPEC-J2). Toxins 2016, 8, 345. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef]
- Ying, C.; Hong, W.; Nianhui, Z.; Chunlei, W.; Kehe, H.; Cuiling, P. Nontoxic concentrations of OTA aggravate DON-induced intestinal barrier dysfunction in IPEC-J2 cells via activation of NF-κB signaling pathway. Toxicol. Lett. 2019, 311, 114–124. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.-P.; Del Rio, J.-C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.-P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Prieto, P.; Graepel, R.; Gerloff, K.; Lamon, L.; Sachana, M.; Pistollato, F.; Gribaldo, L.; Bal-Price, A.; Worth, A. Investigating cell type specific mechanisms contributing to acute oral toxicity. ALTEX 2019, 36, 39–64. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, G. Impact of deoxynivalenol and kaempferol on expression of tight junction proteins at different stages of Caco-2 cell proliferation and differentiation. RSC Adv. 2019, 9, 34607–34616. [Google Scholar] [CrossRef] [PubMed]
- Weidner, M.; Hüwel, S.; Ebert, F.; Schwerdtle, T.; Galla, H.-J.; Humpf, H.-U. Influence of T-2 and HT-2 Toxin on the Blood-Brain Barrier In Vitro: New Experimental Hints for Neurotoxic Effects. PLoS ONE 2013, 8, e60484. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E. Claudins form ion-selective channels in the paracellular pathway. Focus on “Claudin extracellular domains determine paracellular charge selectively and resistance but not tight junction fibril architecture”. Am. J. Physiol. Cell Physiol. 2003, 284, C1331–C1333. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. The Role of Claudins in Determining Paracellular Charge Selectivity. Proc. Am. Thorac. Soc. 2004, 1, 38–41. [Google Scholar] [CrossRef]
- Cummins, P.M. Occludin: One Protein, Many Forms. Mol. Cell. Biol. 2012, 32, 242–250. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Sordillo, L.M. Mammary Gland Immunobiology and Resistance to Mastitis. Vet.-Clin. N. Am. Food Anim. Pract. 2018, 34, 507–523. [Google Scholar] [CrossRef]
- Hou, L.; Le, G.; Lin, Z.; Qian, G.; Gan, F.; Gu, C.; Jiang, S.; Mu, J.; Ge, L.; Huang, K. Nontoxic concentration of ochratoxin A decreases the dosage of cyclosporine A to induce chronic nephropathy model via autophagy mediated by toll-like receptor 4. Cell Death Dis. 2020, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Hao, S.; Gan, F.; Wang, H.; Xu, J.; Liu, D.; Huang, K. In vitro immune toxicity of ochratoxin A in porcine alveolar macrophages: A role for the ROS-relative TLR4/MyD88 signaling pathway. Chem. Biol. Interact. 2017, 272, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Roh, Y.-S.; Cho, A.; Kim, J.; Kim, J.-H.; Eo, S.-K.; Lim, C.-W.; Kim, B. Immune modulatory effects of the foodborne contaminant citrinin in mice. Food Chem. Toxicol. 2012, 50, 3537–3547. [Google Scholar] [CrossRef] [PubMed]
- Cremer, B.; Soja, A.; Sauer, J.-A.; Damm, M. Pro-inflammatory effects of ochratoxin A on nasal epithelial cells. Eur. Arch. Otorhinolaryngol. 2011, 269, 1155–1161. [Google Scholar] [CrossRef]
- Darif, Y.; Mountassif, D.; Belkebir, A.; Zaid, Y.; Basu, K.; Mourad, W.; Oudghiri, M. Ochratoxin A mediates MAPK activation, modulates IL-2 and TNF-α mRNA expression and induces apoptosis by mitochondria-dependent and mitochondria-independent pathways in human H9 T cells. J. Toxicol. Sci. 2016, 41, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Bougarn, S.; Cunha, P.; Gilbert, F.B.; Harmache, A.; Foucras, G.; Rainard, P. Staphylococcal-associated molecular patterns enhance expression of immune defense genes induced by IL-17 in mammary epithelial cells. Cytokine 2011, 56, 749–759. [Google Scholar] [CrossRef]
- Bannerman, D.D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows1. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef]
- Ezzat Alnakip, M.; Quintela-Baluja, M.; Bohme, K.; Fernandez-No, I.; Caamano-Antelo, S.; Calo-Mata, P.; Barros-Velazquez, J. The Immunology of Mammary Gland of Dairy Ruminants between Healthy and Inflammatory Conditions. J. Vet. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef]
- Horwitz, D.A.; Fahmy, T.M.; Piccirillo, C.A.; La Cava, A. Rebalancing Immune Homeostasis to Treat Autoimmune Diseases. Trends Immunol. 2019, 40, 888–908. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Chockalingam, A. Staphylococcus aureus intramammary infection elicits increased production of transforming growth factor-α, β1, and β2. Vet. Immunol. Immunopathol. 2006, 112, 309–315. [Google Scholar] [CrossRef]
- Chockalingam, A.; Paape, M.; Bannerman, D. Increased Milk Levels of Transforming Growth Factor-α, β1, and β2 During Escherichia coli-Induced Mastitis. J. Dairy Sci. 2005, 88, 1986–1993. [Google Scholar] [CrossRef]
- Kauf, A.; Rosenbusch, R.; Paape, M.; Bannerman, D. Innate Immune Response to Intramammary Mycoplasma bovis Infection. J. Dairy Sci. 2007, 90, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Blach-Olszewska, Z.; Leszek, J. Mechanisms of over-activated innate immune system regulation in autoimmune and neurodegenerative disorders. Neuropsychiatr. Dis. Treat. 2007, 3, 365–372. [Google Scholar] [PubMed]
- Datz, C.A. Noninfectious Causes of Immunosuppression in Dogs and Cats. Vet.-Clin. N. Am. Small Anim. Pract. 2010, 40, 459–467. [Google Scholar] [CrossRef]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A. Individual and Combined Effect of Zearalenone Derivates and Beauvericin Mycotoxins on SH-SY5Y Cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Zhao, Y.; Zang, J.; Gao, H. Ochratoxin A induces human kidney tubular epithelial cell apoptosis through regulating lipid raft/ PTEN / AKT signaling pathway. Environ. Toxicol. 2021, 36, 1880–1885. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Boermans, H.J.; Swamy, H.V.; Sharma, B.S.; Karrow, N.A. Immunotoxicity of Penicillium Mycotoxins on Viability and Proliferation of Bovine Macrophage Cell Line (BOMACs). Open Mycol. J. 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Juan-García, A.; Carbone, S.; Ben-Mahmoud, M.; Sagratini, G.; Mañes, J. Beauvericin and ochratoxin A mycotoxins individually and combined in HepG2 cells alter lipid peroxidation, levels of reactive oxygen species and glutathione. Food Chem. Toxicol. 2020, 139, 111247. [Google Scholar] [CrossRef]
- Spevakova, I.; Fernandez-Cruz, M.-L.; Tokarova, K.; Greifova, H.; Capcarova, M. The protective effect of stilbenes resveratrol and pterostilbene individually and combined with mycotoxin citrinin in human adenocarcinoma HT-29 cell line in vitro. J. Environ. Sci. Health Part A 2020, 56, 75–88. [Google Scholar] [CrossRef]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose–response curves. J. Pharmacol. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Majima, A.; Handa, O.; Naito, Y.; Suyama, Y.; Onozawa, Y.; Higashimura, Y.; Mizushima, K.; Morita, M.; Uehara, Y.; Horie, H.; et al. Real-time monitoring of trans-epithelial electrical resistance in cultured intestinal epithelial cells: The barrier protection of water-soluble dietary fiber. J. Dig. Dis. 2017, 18, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.T.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Kumar, P.; Raeman, R.; Liu, Y.; Smith, T.; Anania, F.A. Dysregulation of junctional adhesion molecule-A contributes to ethanol-induced barrier disruption in intestinal epithelial cell monolayers. Physiol. Rep. 2017, 5, e13541. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Gene 1 | Primer Sequence 5′-3′ | PCR Efficiency (%) | Amplicon Length (bp) 2 | Accession No. |
|---|---|---|---|---|
| GAPDH: F | GATGGTGAAGGTCGGAGTGAAC | 101.711 | 100 | NM_001034034.2 |
| GAPDH: R | GTCATTGATGGCGACGATGT | |||
| UXT: F | TTGACACAGTGGTCCCAGAC | 96.603 | 143 | NM_001037471.2 |
| UXT: R | CTTGGTGAGGTTGTCGCTGA | |||
| ZO-1: F | GCGAAATGAGAAACAAGCACC | 97.78 | 121 | XM_024982012.1 |
| ZO-1: R | ATGAGTTGAGTTGGGCAGGAC | |||
| Claudin 3: F | AGGGACTGTGGATGAACTGC | 108.701 | 128 | NM_205801.2 |
| Claudin 3: R | CAGTAGGATGGCGATGACG | |||
| Occludin: F | GCCAGCATATTCCTTCTACCC | 103.487 | 139 | NM_001082433.2 |
| Occludin: R | AAGAGTGGAGGCAACACAGG | |||
| IL-6: F | GGCTCCCATGATTGTGGTAGTT | 105.691 | 523 | NM_173923.2 |
| IL-6: R | GCCCAGTGGACAGGTTTCTG | |||
| TNF-α: F | CGGTGGTGGGACTCGTATG | 103.751 | 352 | NM_173923.2 |
| TNF-α: R | CTGGTTGTCTTCCAGCTTCACA | |||
| TGF-β: F | CCTGAGCCAGAGGCGGACTAC | 99.181 | 130 | NM_001166068.1 |
| TGF-β: R | GCTCGGACGTGTTGAAGAAC | |||
| TLR4: F | GAACAGGTAGCCCAGACAGC | 99.35 | 151 | NM_174198.6 |
| TLR4: R | AGGCCATGATACGGTTGAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Shandilya, U.K.; Yiannikouris, A.; Karrow, N.A. Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function. Toxins 2022, 14, 640. https://doi.org/10.3390/toxins14090640
Xu R, Shandilya UK, Yiannikouris A, Karrow NA. Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function. Toxins. 2022; 14(9):640. https://doi.org/10.3390/toxins14090640
Chicago/Turabian StyleXu, Ran, Umesh K. Shandilya, Alexandros Yiannikouris, and Niel A. Karrow. 2022. "Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function" Toxins 14, no. 9: 640. https://doi.org/10.3390/toxins14090640
APA StyleXu, R., Shandilya, U. K., Yiannikouris, A., & Karrow, N. A. (2022). Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function. Toxins, 14(9), 640. https://doi.org/10.3390/toxins14090640