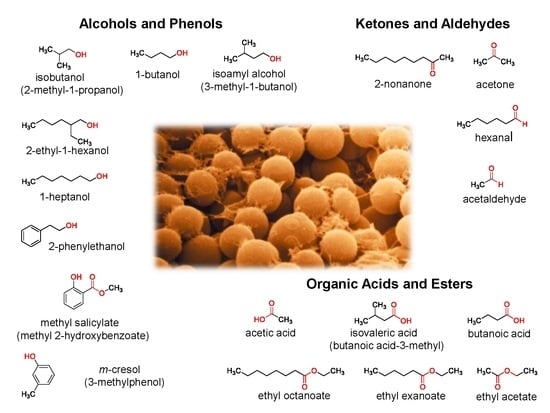
Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi
Abstract
:1. Introduction
2. Yeast Volatilome
3. VOCs-Mediated Control of Postharvest Mycotoxigenic Fungi
3.1. Alternaria
3.2. Aspergillus
3.3. Fusarium
3.4. Penicillium
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prusky, D. Reduction of the incidence of postharvest quality losses, and future prospects. Food Secur. 2011, 3, 463–474. [Google Scholar] [CrossRef]
- Kaminski, J.; Christiaensen, L. Post-harvest loss in sub-Saharan Africa—What do farmers say? Glob. Food Secur. 2014, 3, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Affognon, H.; Mutungi, C.; Sanginga, P.; Borgemeister, C. Unpacking postharvest losses in sub-Saharan Africa: A meta-analysis. World Dev. 2015, 66, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Mwangi, J.K.; Mutungi, C.M.; Midingoyi, S.K.G.; Faraj, A.K.; Affognon, H.D. An assessment of the magnitudes and factors associated with postharvest losses in off-farm grain stores in Kenya. J. Stored Prod. Res. 2017, 73, 7–20. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Yazid, S.N.E.; Jinap, S.; Ismail, S.I.; Magan, N.; Samsudin, N.I.P. Phytopathogenic organisms and mycotoxigenic fungi: Why do we control one and neglect the other? A biological control perspective in Malaysia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 643–669. [Google Scholar] [CrossRef]
- Hymery, N.; Oswald, I.P. Toxicology of Mycotoxins: Overview and Challenges. In Mycotoxins in Food and Beverages, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 187–212. [Google Scholar]
- Dey, D.K.; Kang, J.I.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Simal-Gandara, J.; Xiao, J.; Ali, S.; Huh, Y.S.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Serwah Boateng, N.A.; Ngolong Ngea, G.L.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4906–4930. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a successful development of biocontrol agents: Production, formulation, packaging, and shelf life as key aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Canette, A.; Aymerich, S.; Briandet, R. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, D.; Meiring, S.P.; Bauer, F.F. Modifying Saccharomyces cerevisiae adhesion properties regulates yeast ecosystem dynamics. mSphere 2018, 3, e00383-18. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Opinion of the Scientific Committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 2005, 3, 226. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, M.; Wilson, C.; Droby, S.; Chalutz, E.; El-Ghaouth, A.; Clauzell, S. Postharvest biocontrol: New concepts and applications. In Biological Control. A Global Perspective, 2nd ed.; Vincent, C., Goettel, M.S., Lazarovits, G., Eds.; CAB International: Wallingford, UK, 2007; pp. 262–273. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. Biocontrol of postharvest diseases with antagonistic microorganisms. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 463–498. [Google Scholar]
- Mercier, J.; Jiménez, J.I. Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol. Technol. 2004, 31, 1–8. [Google Scholar] [CrossRef]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a killer: Microbial volatilome and its role in the biological control of plant pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, G.; Dirksie, E.; Sears, J.; Markworth, C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 2001, 147, 2943–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.; Strobel, G.A. Marvellous Muscodor spp.: Update on their biology and applications. Microb. Ecol. 2021, 82, 5–20. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Thongbai, B.; Hyde, K.D.; Brönstrup, M.; Beutling, U.; Lambert, C.; Miller, A.N.; Liu, J.K.M.; Itthayakorn, P.; Stadler, M. Elucidation of the life cycle of the endophytic genus Muscodor and its transfer to Induratia in Induratiaceae fam. nov., based on a polyphasic taxonomic approach. Fungal Divers. 2020, 101, 177–210. [Google Scholar] [CrossRef]
- Braun, G.; Vailati, M.; Prange, R.; Bevis, E. Muscodor albus volatiles control toxigenic fungi under controlled atmosphere (CA) storage conditions. Int. J. Mol. Sci. 2012, 13, 15848–15858. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Meredith, L.K.; Tfaily, M.M. Capturing the microbial volatilome: An oft overlooked'ome'. Trends Microbiol. 2022, 30, 622–631. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 2010, 156, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Hung, R.; Lee, S.; Padhi, S. 18 Fungal and bacterial volatile organic compounds: An overview and their role as ecological signaling agents. In Fungal Associations. The Mycota; Springer: Berlin/Heidelberg, Germany, 2012; pp. 373–393. [Google Scholar]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Mulla, S.I.; Lee, K.J.; Chae, J.C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pollastri, S.; Ruocco, M.; Monti, M.M.; Loreto, F. Volatile organic compounds in the interaction between plants and beneficial microorganisms. J. Plant Interact. 2022, 17, 840–852. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food: Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Agtmaal, M.; Straathof, A.L.; Termorshuizen, A.; Lievens, B.; Hoffland, E.; de Boer, W. Volatile-mediated suppression of plant pathogens is related to soil properties and microbial community composition. Soil Biol. Biochem. 2018, 117, 164–174. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Jousset, A.; Friman, V.P.; Mei, X.; Wang, S.; Wei, Z.; Shen, Q. Bacterial community richness shifts the balance between volatile organic compound-mediated microbe–pathogen and microbe–plant interactions. Proc. Biol. Sci. 2020, 287, 20200403. [Google Scholar] [CrossRef] [Green Version]
- Ezra, D.; Strobel, G.A. Effect of substrate on the bioactivity of volatile antimicrobials produced by Muscodor albus. Plant Sci. 2003, 165, 1229–1238. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Cellini, A.; Spinelli, F.; Donati, I.; Ryu, C.M.; Kloepper, J.W. Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 2021, 26, 968–983. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Ebert, B.E.; Halbfeld, C.; Blank, L.M. Exploration and exploitation of the yeast volatilome. Curr. Metab. 2017, 5, 102–108. [Google Scholar] [CrossRef]
- Dubois, A.; Carrere, S.; Raymond, O.; Pouvreau, B.; Cottret, L.; Roccia, A.; Onesto, J.P.; Sakr, S.; Atanassova, R.; Baudino, S.; et al. Transcriptome database resource and gene expression atlas for the rose. BMC Genom. 2012, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, H.; Wang, Q.; Jian, H.; Qiu, X.; Wang, J.; Tang, K. Isolation and identification of a putative scent-related gene RhMYB1 from rose. Mol. Biol. Rep. 2011, 38, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.K.; Hua, S.; Sarreal, S.; Li, R. Suppression of aflatoxin biosynthesis in Aspergillus flavus by 2-phenylethanol is associated with stimulated growth and decreased degradation of branched-chain amino acids. Toxins 2015, 7, 3887–3902. [Google Scholar] [CrossRef] [Green Version]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Tilocca, B.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. A proteomic investigation of Aspergillus carbonarius exposed to yeast volatilome or to its major component 2-phenylethanol reveals major shifts in fungal metabolism. Int. J. Food Microbiol. 2019, 306, 108265. [Google Scholar] [CrossRef]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Masoud, W.; Poll, L.; Jakobsen, M. Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Yeast 2005, 22, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Masoud, W.; Kaltoft, C.H. The effects of yeasts involved in the fermentation of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus. Int. J. Food Microbiol. 2006, 106, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Hatanaka, K.; Ohata, I.; Yamashita-Kitaguchi, Y.; Kurata, A.; Kishimoto, N. Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control 2012, 26, 472–478. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie van Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef]
- Alasmar, R.; Ul-Hassan, Z.; Zeidan, R.; Al-Thani, R.; Al-Shamary, N.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Isolation of a novel Kluyveromyces marxianus strain QKM-4 and evidence of its volatilome production and binding potentialities in the biocontrol of toxigenic fungi and their mycotoxins. ACS Omega 2020, 5, 17637–17645. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Arrarte, E.; Garmendia, G.; Rossini, C.; Wisniewski, M.; Vero, S. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Zhou, Y.J. Expanding the terpenoid kingdom. Nat. Chem. Biol. 2018, 14, 1069–1070. [Google Scholar] [CrossRef]
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum sensing in fungal species. Annu. Rev. Microbiol. 2021, 75, 449–469. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zebec, Z.; Wilkes, J.; Jervis, A.J.; Scrutton, N.S.; Takano, E.; Breitling, R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 2016, 34, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, L.; Zhang, M.; Wei, Y.; Lin, W. Recent advances on feasible strategies for monoterpenoid production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 609800. [Google Scholar] [CrossRef] [PubMed]
- Barkai-Golan, R. Alternaria mycotoxins. In Mycotoxins in Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2008; pp. 185–203. [Google Scholar]
- Patriarca, A. Alternaria in food products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, J.; Wu, J.; Wang, L.; et al. Alternaria mycotoxins: An overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Fleck, S.C.; Burkhardt, B.; Pfeiffer, E.; Metzler, M. Alternaria toxins: Altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol. Lett. 2012, 214, 27–32. [Google Scholar] [CrossRef]
- Sharma, N.; Verma, U.; Awasthi, P. A combination of the yeast Candida utilis and chitosan controls fruit rot in tomato caused by Alternaria alternata (Fr.) Keissler and Geotrichum candidum Link ex Pers. Hortic. Sci. Biotechnol. 2006, 81, 1043–1051. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Y.; Shen, D.; Feng, W.; Yu, T.; Zhang, J.; Zheng, X.D. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int. J. Food Microbiol. 2008, 123, 234–239. [Google Scholar]
- Prendes, L.P.; Merín, M.G.; Zachetti, V.G.L.; Pereyra, A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Impact of antagonistic yeasts from wine grapes on growth and mycotoxin production by Alternaria alternata. J. Appl. Microbiol. 2021, 131, 833–843. [Google Scholar] [CrossRef]
- Leng, J.; Dai, Y.; Qiu, D.; Zou, Y.; Wu, X. Utilization of the antagonistic yeast, Wickerhamomyces anomalus, combined with UV-C to manage postharvest rot of potato tubers caused by Alternaria tenuissima. Int. J. Food Microbiol. 2022, 377, 109782. [Google Scholar] [CrossRef] [PubMed]
- Fiori, S.; Urgeghe, P.P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Rodriguez Assaf, L.A.; Toro, M.E.; Castellanos de Figueroa, L.I.; Vazquez, F. Antifungal modes of action of Saccharomyces and other biocontrol yeasts against fungi isolated from sour and grey rots. Int. J. Food Microbiol. 2015, 204, 91–100. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.L.; Ribeiro, L.S.; Miguel, M.G.D.C.P.; Batista, L.R.; Schwan, R.F.; Medeiros, F.H.; Silva, C.F. Yeasts prevent ochratoxin A contamination in coffee by displacing Aspergillus carbonarius. Biol. Control 2021, 155, 104512. [Google Scholar] [CrossRef]
- Zeidan, R.; Ul-Hassan, Z.; Al-Thani, R.; Balmas, V.; Jaoua, S. Application of low-fermenting yeast Lachancea thermotolerans for the control of toxigenic fungi Aspergillus parasiticus, Penicillium verrucosum and Fusarium graminearum and their mycotoxins. Toxins 2018, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Rohani, M.; Fani, S.R.; Mosavian, M.T.H.; Probst, C.; Khodaygan, P. Biocontrol potential of native yeast strains against Aspergillus flavus and aflatoxin production in Pistachio. Food Addit. Contam. 2020, 37 Pt A, 1963–1973. [Google Scholar] [CrossRef]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. BioControl 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Effects of temperature and relative humidity on Aflatoxin B1 reduction in corn grains and antagonistic activities against aflatoxin-producing Aspergillus flavus by a volatile organic compound-producing yeast, Kwoniella heveanensis DMKU-CE82. BioControl 2021, 66, 433–443. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Tejero, P.; Martín, A.; Rodríguez, A.; Galván, A.I.; Ruiz-Moyano, S.; Hernández, A. In vitro biological control of Aspergillus flavus by Hanseniaspora opuntiae L479 and Hanseniaspora uvarum L793, producers of antifungal volatile organic compounds. Toxins 2021, 13, 663. [Google Scholar] [CrossRef]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Velazhahan, R.; Paranidharan, V. Volatiles of antagonistic soil yeasts inhibit growth and aflatoxin production of Aspergillus flavus. Microbiol. Res. 2022, 263, 127150. [Google Scholar] [CrossRef] [PubMed]
- Galván, A.I.; Hernández, A.; de Guia Córdoba, M.; Martín, A.; Serradilla, M.J.; López-Corrales, M.; Rodríguez, A. Control of toxigenic Aspergillus spp. In dried figs by volatile organic compounds (VOCs) from antagonistic yeasts. Int. J. Food Microbiol. 2022, 376, 109772. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H.; Gilbert, M.K. The potential role of fungal volatile organic compounds in Aspergillus flavus biocontrol efficacy. Biol. Control 2021, 160, 104686. [Google Scholar] [CrossRef]
- Yang, T.; Wang, C.; Li, C.; Sun, R.; Yang, M. Antagonistic effects of volatile organic compounds of Saccharomyces cerevisiae NJ-1 on the growth and toxicity of Aspergillus flavus. Biol. Control 2023, 177, 105093. [Google Scholar] [CrossRef]
- Alkuwari, A.; Hassan, Z.U.; Zeidan, R.; Al-Thani, R.; Jaoua, S. Occurrence of mycotoxins and toxigenic fungi in cereals and application of yeast volatiles for their biological control. Toxins 2022, 14, 404. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Biological Control of Fusarium culmorum, Fusarium graminearum and Fusarium poae by antagonistic yeasts. Pathogens 2022, 11, 86. [Google Scholar] [CrossRef]
- Björnberg, A.; Schnürer, J. Inhibition of the growth of grain-storage molds in vitro by the yeast Pichia anomala (Hansen) Kurtzman. Can. J. Microbiol. 1993, 39, 623–628. [Google Scholar] [CrossRef]
- Ädel Druvefors, U.; Schnürer, J. Mold-inhibitory activity of different yeast species during airtight storage of wheat grain. FEMS Yeast Res. 2005, 5, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Ädel Druvefors, U.A.; Passoth, V.; Schnürer, J. Nutrient effects on biocontrol of Penicillium roqueforti by Pichia anomala J121 during airtight storage of wheat. Appl. Environ. Microbiol. 2005, 71, 1865–1869. [Google Scholar] [CrossRef] [Green Version]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, L.; Johansen, P.G.; Petersen, M.A.; Arneborg, N.; Jespersen, L. Debaryomyces hansenii strains isolated from Danish cheese brines act as biocontrol agents to inhibit germination and growth of contaminating molds. Front. Microbiol. 2021, 12, 662785. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Martini, C.; Spadoni, A.; Rouissi, W.; Bertolini, P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol. Technol. 2012, 73, 56–62. [Google Scholar] [CrossRef]
- Lutz, M.C.; Lopes, C.A.; Rodriguez, M.E.; Sosa, M.C.; Sangorrín, M.P. Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int. J. Food Microbiol. 2013, 164, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Agirman, B.; Erten, H. Biocontrol ability and action mechanisms of Aureobasidium pullulans GE17 and Meyerozyma guilliermondii KL3 against Penicillium digitatum DSM2750 and Penicillium expansum DSM62841 causing postharvest diseases. Yeast 2020, 37, 437–448. [Google Scholar] [CrossRef]
- Gomomo, Z.; Fanadzo, M.; Mewa-Ngongang, M.; Hoff, J.; Van der Rijst, M.; Okudoh, V.; Kriel, J.; du Plessis, H. Control of mould spoilage on apples using yeasts as biological control agents. Pol. J. Food Nutr. Sci. 2022, 72, 119–128. [Google Scholar] [CrossRef]
- Yalage Don, S.M.Y.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Volatile organic compounds produced by Aureobasidium pullulans induce electrolyte loss and oxidative stress in Botrytis cinerea and Alternaria alternata. Res. Microbiol. 2021, 172, 103788. [Google Scholar] [CrossRef]
- Fialho, M.B.; Carvalho, G.; Martins, P.F.; Azevedo, R.A.; Pascholati, S.F. Antioxidative response of the fungal plant pathogen Guignardia citricarpa to antimicrobial volatile organic compounds. Afr. J. Microbiol. Res. 2014, 8, 2077–2084. [Google Scholar]
- Rezende, D.C.; Fialho, M.B.; Brand, S.C.; Blumer, S.; Pascholati, S.F. Antimicrobial activity of volatile organic compounds and their effect on lipid peroxidation and electrolyte loss in Colletotrichum gloeosporioides and Colletotrichum acutatum mycelia. Afr. J. Microbiol. Res. 2015, 9, 1527–1535. [Google Scholar]
- Barkai-Golan, R. Aspergillus mycotoxins. In Mycotoxins in Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2008; pp. 115–151. [Google Scholar]
- Frisvad, J.C.; Larsen, T.O. Chemodiversity in the genus Aspergillus. Appl. Microbiol. Biotechnol. 2015, 99, 7859–7877. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical agents and related occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 9. [Google Scholar]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Frisvad, J.C.; Thrane, U.; Nielsen, K.F. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J. Agric. Food Chem. 2010, 58, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and ochratoxin production in industrial Aspergillus niger strains. PLoS ONE 2011, 6, e23496. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Pasquali, M.; Migheli, Q. Genetic approaches to chemotype determination in type B trichothecene producing Fusaria. Int. J. Food Microbiol. 2014, 189, 164–182. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod Process Nutr. 2019, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cendoya, E.; Chiotta, M.L.; Zachetti, V.; Chulze, S.N.; Ramirez, M.L. Fumonisins and fumonisin-producing Fusarium occurrence in wheat and wheat by products: A review. J. Cereal Sci. 2018, 80, 158–166. [Google Scholar] [CrossRef]
- Richard-Forget, F.; Atanasova, V.; Chéreau, S. Using metabolomics to guide strategies to tackle the issue of the contamination of food and feed with mycotoxins: A review of the literature with specific focus on Fusarium mycotoxins. Food Control 2021, 121, 107610. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Penicillium mycotoxins. In Mycotoxins in Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2008; pp. 153–183. [Google Scholar]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, D.M.; Müller, C.; Metzler, M.; Lehmann, L. DNA–DNA cross-links contribute to the mutagenic potential of the mycotoxin patulin. Toxicol. Lett. 2006, 166, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Susca, A. Penicillium species and their associated mycotoxins. Mycotoxigenic Fungi. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1542, pp. 107–119. [Google Scholar]
- Qureshi, N.; Tamhane, D.V. Production of mead by immobilized cells of Hansenula anomala. Appl. Microbiol. Biotechnol. 1987, 27, 27–30. [Google Scholar] [CrossRef]
- Saksena, N.; Tripathi, H.H. Effect of organic volatiles from Saccharomyces on the spore germination of fungi. Acta Microbiol. Hung. 1987, 34, 255–257. [Google Scholar]
- Castoria, R.; Wright, S.A.; Droby, S. Biological control of mycotoxigenic fungi in fruits. In Mycotoxins in Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2008; pp. 311–333. [Google Scholar]
- Castoria, R.; Morena, V.; Caputo, L.; Panfili, G.; De Curtis, F.; De Cicco, V. Effect of the biocontrol yeast Rhodotorula glutinis strain LS11 on patulin accumulation in stored apples. Phytopathology 2005, 95, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Castoria, R.; Mannina, L.; Durán-Patrón, R.; Maffei, F.; Sobolev, A.P.; De Felice, D.V.; Cristina Pinedo-Rivilla, C.; Ritieni, A.; Ferracane, R.; Wright, S.A. Conversion of the mycotoxin patulin to the less toxic desoxypatulinic acid by the biocontrol yeast Rhodosporidium kratochvilovae strain LS11. J. Agric. Food Chem. 2011, 59, 11571–11578. [Google Scholar] [CrossRef]
- Ianiri, G.; Idnurm, A.; Castoria, R. Transcriptomic responses of the basidiomycete yeast Sporobolomyces sp. to the mycotoxin patulin. BMC Genom. 2016, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhou, T.; Wu, T.; Li, X. Saccharomyces cerevisiae YE-7 reduces the risk of apple blue mold disease by inhibiting the fungal incidence and patulin biosynthesis. J. Food Process. Preserv. 2018, 42, e13360. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Zhang, Z.; Yuan, Y.; Yue, T. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Pennerman, K.K.; Scarsella, J.B.; Yin, G.H.; Hua, S.S.T.; Hartman, T.G.; Bennett, J.W. Volatile 1-octen-3-ol increases patulin production by Penicillium expansum on a patulin-suppressing medium. Mycotoxin Res. 2019, 35, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana-Tachon, A.; Strub, C.; Schorr-Galindo, S. Apple-based coatings incorporated with wild apple isolated yeast to reduce Penicillium expansum postharvest decay of apples. Postharvest Biol. Technol. 2022, 185, 111805. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L.T. Triggered and controlled release of active gaseous/volatile compounds for active packaging applications of agri-food products: A review. Compr. Rev. Food Sci. Food Saf. 2022, 2, 541–579. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiol. 2017, 63, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Almenar, E.; Auras, R.; Rubino, M.; Harte, B. A new technique to prevent the main post harvest diseases in berries during storage: Inclusion complexes β-cyclodextrin-hexanal. Int. J. Food Microbiol. 2007, 118, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Martini, A.; Cappelli, F.; Pagnoni, U.M.; Davoli, P. A study on volatile organic compounds (VOCs) produced by tropical ascomycetous yeasts. Antonie van Leeuwenhoek 2003, 84, 301–311. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Gamir, J.; Larraya, L.; Farran, I.; Veramendi, J. Towards understanding of fungal biocontrol mechanisms of different yeasts antagonistic to Botrytis cinerea through exometabolomic analysis. Biol. Control 2022, 174, 105033. [Google Scholar] [CrossRef]
- Gasperini, A.M.; Rodriguez-Sixtos, A.; Verheecke-Vaessen, C.; Garcia-Cela, E.; Medina, A.; Magan, N. Resilience of biocontrol for aflatoxin minimization strategies: Climate change abiotic factors may affect control in non-GM and GM-maize cultivars. Front. Microbiol. 2019, 10, 2525. [Google Scholar] [CrossRef]
- Magan, N.; Gasperini Marcon, A.; Putra Samsudin, N.I.; Rodríguez-Sixtos, A.; Garcia-Cela, E.; Verheecke-Vaessen, C.; Medina, A. Biological control agents for mycotoxin control: Are they resilient enough? In How Research Can Stimulate the Development of Commercial Biological Control against Plant Diseases; Springer: Cham, Switzerland, 2020; pp. 295–309. [Google Scholar]
- Tan, M.; Caro, Y.; Sing, A.S.C.; Reiss, H.; Francois, J.M.; Petit, T. Selection by UV mutagenesis and physiological characterization of mutant strains of the yeast Saprochaete suaveolens (Former Geotrichum fragrans) with higher capacity to produce flavor compounds. J. Fungi 2021, 7, 1031. [Google Scholar] [CrossRef]
- Tufariello, M.; Rizzuti, A.; Palombi, L.; Ragone, R.; Capozzi, V.; Gallo, V.; Mastrorilli, P.; Grieco, F. Non-targeted metabolomic approach as a tool to evaluate the chemical profile of sparkling wines fermented with autochthonous yeast strains. Food Control 2021, 126, 108099. [Google Scholar] [CrossRef]
- Ellis, D.J.; Kerr, E.D.; Schenk, G.; Schulz, B.L. Metabolomics of non-Saccharomyces yeasts in fermented beverages. Beverages 2022, 8, 41. [Google Scholar] [CrossRef]
- Liu, J.; Clarke, J.A.; McCann, S.; Hillier, N.K.; Tahlan, K. Analysis of Streptomyces volatilomes using global molecular networking reveals the presence of metabolites with diverse biological activities. Microbiol. Spectr. 2022, 4, e00552-22. [Google Scholar] [CrossRef] [PubMed]
| Target Fungus | Mycotoxin | Major Volatile(s) | Emitting Yeast | Mechanism/Function Inhibited | Commodity or Experimental Setup | Reference |
|---|---|---|---|---|---|---|
| Alternaria alternata, A. arborescens, A. tenuissima | N.D. | 3-methylbutyl hexanoate, 3-methylbutylpentanoate, 2-methylpropyl hexanoate, pentylhexanoate | Candida sake | Vegetative growth, infection and fruit rot | In vitro and apple fruit | [72] |
| Alternaria alternata | N.D. | ethyl acetate, isoamyl acetate, ethyl butyrate, ethyl hexanoate, phenylethyl acetate, 2-phenylethanol, isobutanol | Metschnikowia pulcherrima, Saccharomyces cerevisiae, Wickerhamomyces anomalus (syn. Pichia anomala) | Vegetative growth, infection and fruit rot | In vitro and strawberry fruit | [67] |
| Alternaria alternata | N.D. | ethanol, 2-methyl-1-propanol, 3-methyl-1-butanol, 2-phenylethanol | Aureobasidium pullulans | Spore germination, vegetative growth, membrane permeability, cell wall integrity | In vitro | [63,78] |
| Aspergillus ochraceus | OTA | ethyl acetate, isobutyl acetate, 2-phenyl ethyl acetate, ethyl propionate, isoamyl alcohol | Pichia anomala, Pichia kluyveri, Hanseniaspora uvarum | Spore germination, vegetative growth, OTA biosynthesis | In vitro | [64,65] |
| Aspergillus carbonarius | OTA | N.D. | Candida friedrichii, Candida intermedia, Cyberlindnera jadinii, Lachancea thermotolerans | Vegetative growth, sporulation, infection, OTA biosynthesis | In vitro and grape berries | [87] |
| Aspergillus carbonarius, A. caelatus A. terreus, A. versicolor | N.D. | N.D. | Saccharomyces cerevisiae, S. kluyveri, Candida sake, Schwanniomyces vanrijiae, Wickerhamiella versatilis (Syn. Candida versatilis), | Vegetative growth | In vitro | [88] |
| Aspergillus carbonarius, A. ochraceus | OTA | 2-phenylethanol | Candida friedrichii, Candida intermedia, Cyberlindnera jadinii, Lachancea thermotolerans | Vegetative growth, sporulation, gene expression, OTA biosynthesis | In vitro, in silico | [61] |
| Aspergillus carbonarius | OTA | 2-phenylethanol | Candida intermedia | Vegetative growth, OTA biosynthesis, fungal metabolism | In vitro, in silico, proteome analysis | [62] |
| Aspergillus carbonarius | OTA | nonadecane, eicosane, docosane, heptacosane, hexatriacontane, and tetracosane | Kluyveromyces marxianus | OTA biosynthesis, infection | In vitro and grape berries | [70] |
| Aspergillus carbonarius, A. ochraceus | OTA | 2-methyl-1-butanol, 3-methyl-1-butanol, 3-methylbutyl acetate, ethyl octanoate, 2-nonanone | Saccharomyces cerevisiae | OTA biosynthesis, infection | In vitro and coffee beans | [89] |
| Aspergillus flavus | AFs | 2-phenylethanol | Pichia anomala | Spore germination, vegetative growth, AF biosynthesis, gene expression | In vitro, in silico | [57] |
| Aspergillus flavus | N.D. | 2-phenylethanol | Wickerhamomyces anomalus (syn. Pichia anomala) | Vegetative growth, AF biosynthesis, gene expression | In vitro, in silico | [59] |
| Aspergillus parasiticus | AFs | 2-phenylethanol | Lachancea thermotolerans | Vegetative growth, AF biosynthesis | In vitro | [90] |
| Aspergillus flavus | N.D. | 2-phenylethanol, phenylethyl acetate, several esters, alcohols, terpenes, ketones, aldehydes, and aromatic hydrocarbons | Issatchenkia orientalis (syn. Pichia kudriavzevii), Pichia occidentalis, Meyerozyma guilliermondii, Meyerozyma caribbica | Vegetative growth | In vitro | [69] |
| Aspergillus flavus | AFB1 | N.D. | Issatchenkia orientalis (syn. Pichia kudriavzevii), Lachancea thermotolerans | Vegetative growth, AF biosynthesis | In vitro | [91] |
| Aspergillus flavus | AFB1 | 1-pentanol | Candida nivariensis | Spore germination, vegetative growth, AF biosynthesis | In vitro | [92] |
| Aspergillus flavus | AFB1 | 3-methyl-1-butanol, 2-methyl-1-butanol, hydrazine-1-1-dimethyl, butanoic acid-3-methyl | Kwoniella heveanensis | Vegetative growth, sporulation, AF biosynthesis | In vitro and maize kernels | [93] |
| Aspergillus flavus | AFs | N.D. | Candida friedrichii, Candida intermedia, Cyberlindnera jadinii, Lachancea thermotolerans | Vegetative growth, AF biosynthesis, gene expression | In vitro, in silico | [94] |
| Aspergillus flavus | AFB1, AFB2 | acetic acid, 2-methylbutanoic acid, isobutyric acid, 2-methylbutanol, isoamyl alcohol, 2-methyl-1-butanol, 2-phenylethanol, ethyl acetate, isoamyl acetate, 2-phenylethyl acetate, 2-methylbutyl acetate | Hanseniaspora opuntiae, Hanseniaspora uvarum | Vegetative growth, AF biosynthesis, gene expression | In vitro, in silico | [95] |
| Aspergillus flavus | AFB1 | 1-pentanol, 1-propanol, ethyl hexanol, ethanol, 2-methyl-1-butanol, ethyl acetate, dimethyl trisulfide, p-xylene, styrene, 1,4-pentadiene, ethyl acetate, hexanal, 1-propanol, 1-heptanol, 1-butanol, benzothiazole | Candida tropicalis Issatchenkia orientalis (syn. Pichia kudriavzevii), Saccharomyces cerevisiae, Suhomyces xylopsoci | Vegetative growth, AF biosynthesis | In vitro | [96] |
| Aspergillus flavus, A. niger | OTA, AFs | furfuryl acetate, 2-phenylethyl acetate | Hanseniaspora opuntiae, Hanseniaspora uvarum | Spore germination, vegetative growth, infection, OTA and AF biosynthesis, gene expression | In vitro, in silico, and dried figs | [97] |
| Aspergillus flavus | N.D. | N.D. | Issatchenkia orientalis (syn. Pichia kudriavzevii), Saccharomyces cerevisiae | Spore germination, vegetative growth | In vitro | [98] |
| Aspergillus flavus | AF | acetaldehyde, 2 ethyl acetate, ethanol, 1-propanol, 2-methyl-1-propanol, 3-methyl-1-butanol, 2-pentanol, acetic acid, 2-phenyl ethanol | Saccharomyces cerevisiae | Spore germination, vegetative growth, sporulation, infection cell membrane permeability, cell wall integrity, AF biosynthesis | In vitro and walnut | [99] |
| Aspergillus parasiticus, A. niger, A. verrucosum | N.D. | 2-phenylethanol | Cyberlindnera jadinii | Vegetative growth | In vitro | [100] |
| Fusarium proliferatum, F. subglutinans | N.D. | N.D. | Debaryomyces hansenii | Vegetative growth | In vitro | [101] |
| Fusarium graminearum | DON | 2-phenylethanol | Lachancea thermotolerans | Vegetative growth, DON biosynthesis | In vitro | [90] |
| Fusarium cerealis, F. poae | N.D. | 2-phenylethanol, phenylethyl acetate, several esters, alcohols, terpenes, ketones, aldehydes, and aromatic hydrocarbons | Issatchenkia orientalis (syn. Pichia kudriavzevii), Pichia occidentalis, Meyerozyma guilliermondii, Meyerozyma caribbica | Vegetative growth | In vitro | [69] |
| Fusarium culmorum, F. graminearum, F. poae | N.D. | N.D. | Meyerozyma guilliermondii Cyberlindnera saturnus, Rhodotorula glutinis, Cryptococcus carnescens | Vegetative growth | In vitro | [102] |
| Penicillium roqueforti, Aspergillus candidus | ethyl acetate | Pichia anomala | Vegetative growth | In vitro | [103] | |
| Penicillium roqueforti | N.D. | ethanol, ethyl acetate | Pichia anomala | Infection | Wheat grain | [104,105] |
| Penicillium citrinum, Penicillium chrysogenum | N.D. | isoamyl acetate, isoamyl alcohol | Candida maltosa | Spore germination | In vitro | [66] |
| Penicillium verrucosum | N.D. | 2-methyl-1-propanol, 3-methyl-1-butanol and 2-methyl-1-butanol | Debaryomyces hansenii | Vegetative growth | In vitro | [106] |
| Penicillium chrysogenum, Penicillium expansum | N.D. | 2-phenylethanol, phenylethyl acetate, several esters, alcohols, terpenes, ketones, aldehydes, and aromatic hydrocarbons | Issatchenkia orientalis (syn. Pichia kudriavzevii), Pichia occidentalis, Meyerozyma guilliermondii, Meyerozyma caribbica | Vegetative growth | In vitro | [69] |
| Penicillium verrucosum | OTA | nonadecane, eicosane, docosane, heptacosane, hexatriacontane, and tetracosane | Kluyveromyces marxianus | OTA biosynthesis, vegetative growth | In vitro | [70] |
| Penicillium roqueforti | N.D. | 2-phenylethanol, acetone | Debaryomyces hansenii | Spore germination, vegetative growth | In vitro | [107] |
| Penicillium expansum | N.D. | N.D. | Aureobasidium pullulans | Vegetative growth | In vitro | [108] |
| Penicillium expansum | N.D. | N.D. | Cryptococcus victoriae, Naganishia albida (syn. C. albidus) | Spore germination, vegetative growth, infection | In vitro and pear fruit | [109] |
| Penicillium expansum | N.D. | 3-methyl-1-butanol, 2-methyl-1-butanol, 2-methyl-1-propanol, 2-phenylethanol | Aureobasidium pullulans | Spore germination, vegetative growth, infection | In vitro and apple fruit | [60] |
| Penicillium expansum | N.D. | N.D. | Aureobasidium pullulans; Meyerozyma guilliermondii | Vegetative growth | In vitro | [110] |
| Penicillium expansum | N.D. | N.D. | Candida pyralidae, Meyerozyma guilliermondii, Pichia kluyveri | Vegetative growth | In vitro | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oufensou, S.; Ul Hassan, Z.; Balmas, V.; Jaoua, S.; Migheli, Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins 2023, 15, 45. https://doi.org/10.3390/toxins15010045
Oufensou S, Ul Hassan Z, Balmas V, Jaoua S, Migheli Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins. 2023; 15(1):45. https://doi.org/10.3390/toxins15010045
Chicago/Turabian StyleOufensou, Safa, Zahoor Ul Hassan, Virgilio Balmas, Samir Jaoua, and Quirico Migheli. 2023. "Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi" Toxins 15, no. 1: 45. https://doi.org/10.3390/toxins15010045
APA StyleOufensou, S., Ul Hassan, Z., Balmas, V., Jaoua, S., & Migheli, Q. (2023). Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins, 15(1), 45. https://doi.org/10.3390/toxins15010045