Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models
Abstract
:1. Introduction
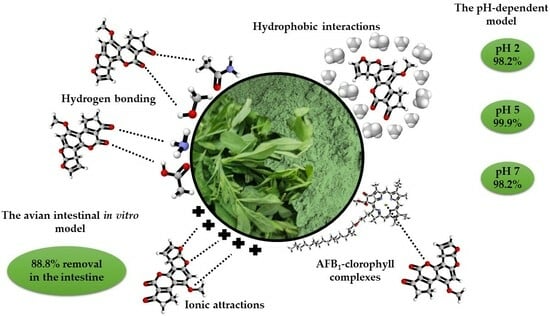
2. Results and Discussion
2.1. Adsorption Experiments
2.1.1. The pH-Dependent Model
2.1.2. The Avian Intestinal Model
2.2. Characterization
2.2.1. FTIR-ATR
2.2.2. ESEM
2.2.3. XRF
2.2.4. XRD
2.2.5. pHpzc and ζ-Potential
2.2.6. Determination of Chlorophylls and Carotenoids
Spectral Reflectance Measurements
Quantitative Determination of Chlorophylls and Carotenoids
3. The Proposed Adsorption Mechanism
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Adsorbent Materials
5.3. In Vitro Adsorption Studies
5.3.1. Preparation of the Aflatoxin B1 (AFB1) Solution and the pH-Dependent Model
5.3.2. Preparation of the AFB1-Contaminated Diet and the Avian Intestinal Model
5.3.3. Analysis of Aflatoxin B1 (AFB1)
5.4. Characterization
5.4.1. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR)
5.4.2. Environmental Scanning Electron Microscopy (ESEM)
5.4.3. X-ray Fluorescence Spectroscopy (XRF)
5.4.4. X-ray Diffraction (XRD)
5.4.5. Point of Zero Charge (pHpzc) and Zeta Potential (ζ-Potential)
5.4.6. Chlorophyll and Carotenoid Quantification
5.5. Experimental Design and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaynes, W.F.; Zartman, R.E.; Hudnall, W.H. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay Sci. 2007, 36, 197–205. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X. Emerging strategies in fluorescent aptasensor toward food hazard aflatoxins detection. Trends Food Sci. Technol. 2022, 129, 621–633. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, S.; Peng, X.; Geng, J.; Chen, C.; Li, M. Al–Fe PILC preparation, characterization and its potential adsorption capacity for aflatoxin B1. Appl. Clay Sci. 2013, 83–84, 231–237. [Google Scholar] [CrossRef]
- Meneely, J.P.; Kolawole, O.; Haughey, S.A.; Miller, S.J.; Krska, R.; Elliott, C.T. The Challenge of Global Aflatoxins Legislation with a Focus on Peanuts and Peanut Products: A Systematic Review. Expo. Health 2022, 15, 467–487. [Google Scholar] [CrossRef]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Available online: http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=WHOLIS&lang=p&nextAction=lnk&exprSearch=9283212568&indexSearch=ID (accessed on 7 September 2023).
- Van Rensburg, C.J.; Van Rensburg, C.E.J.; Van Ryssen, J.B.J.; Casey, N.H.; Rottinghaus, G.E. In Vitro and In Vivo Assessment of Humic Acid as an Aflatoxin Binder in Broiler Chickens. Poult. Sci. 2006, 85, 1576–1583. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; de la Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zuniga, K.; Laurie, V.F.; Moore-Carrasco, R.; Ortiz-Villeda, B.; Carrasco-Sánchez, V. Agro-industrial Waste Products as Mycotoxin Biosorbents: A Review of In Vitro and In Vivo Studies. Food Rev. Int. 2021, 39, 2914–2930. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; García-Rosales, G.; Longoria-Gándara, L.; Gómez-Vilchis, J. Obtention of biochar-Ca nanoparticles using Citrus tangerina׃ A morphological, surface and study remotion of Aflatoxin AFB1. J. Hazard. Mater. 2021, 424, 127339. [Google Scholar] [CrossRef]
- Zavala-Franco, A.; Hernández-Patlán, D.; Solís-Cruz, B.; López-Arellano, R.; Tellez-Isaias, G.; Vázquez-Durán, A.; Méndez-Albores, A. Assessing the Aflatoxin B1 Adsorption Capacity between Biosorbents Using an In Vitro Multicompartmental Model Simulating the Dynamic Conditions in the Gastrointestinal Tract of Poultry. Toxins 2018, 10, 484. [Google Scholar] [CrossRef]
- Ramales-Valderrama, R.A.; Vázquez-Durán, A.; Méndez-Albores, A. Biosorption of B-aflatoxins Using Biomasses Obtained from Formosa Firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins 2016, 8, 218. [Google Scholar] [CrossRef]
- Vázquez-Durán, A.; Nava-Ramírez, M.d.J.; Téllez-Isaías, G.; Méndez-Albores, A. Removal of Aflatoxins Using Agro-Waste-Based Materials and Current Characterization Techniques Used for Biosorption Assessment. Front. Vet. Sci. 2022, 9, 897302. [Google Scholar] [CrossRef]
- Rasheed, U.; Ain, Q.U.; Yaseen, M.; Santra, S.; Yao, X.; Liu, B. Assessing the Aflatoxins Mitigation Efficacy of Blueberry Pomace Biosorbent in Buffer, Gastrointestinal Fluids and Model Wine. Toxins 2020, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, A.; Şentürk, E.; Olgun, O. Use of alfalfa meal in layer diets—A review. Worlds Poult. Sci. J. 2020, 76, 134–143. [Google Scholar] [CrossRef]
- Samur, S.I.N.; Suwignyo, B.; Suryanto, E. The effect of Alfalfa (Medicago sativa L.) on different basal feeds for hybrid duck performance. E3S Web Conf. 2020, 200, 03013. [Google Scholar] [CrossRef]
- Koçer, B.; Bozkurt, M.; Ege, G.; Tüzün, A.E.; Konak, R.; Olgun, O. Effects of a meal feeding regimen and the availability of fresh alfalfa on growth performance and meat and bone quality of broiler genotypes. Br. Poult. Sci. 2018, 59, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Suwignyo, B.; Sasongko, H. The effect of fresh and hay alfalfa (Medicago sativa L.) supplementation on hybrid duck performance. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012085. [Google Scholar] [CrossRef]
- Suwignyo, B.; Rini, E.A.; Fadli, M.K.; Ariyadi, B. Effects of alfalfa (Medicago sativa L.) supplementation in the diet on the growth, small intestinal histomorphology, and digestibility of hybrid ducks. Vet. World 2021, 14, 2719. [Google Scholar] [CrossRef] [PubMed]
- Pliego, A.B.; Tavakoli, M.; Khusro, A.; Seidavi, A.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Márquez-Molina, O.; Rivas-Caceres, R.R. Beneficial and adverse effects of medicinal plants as feed supplements in poultry nutrition: A review. Anim. Biotechnol. 2020, 33, 369–391. [Google Scholar] [CrossRef]
- Shahsavari, K. Influences of different sources of natural pigments on the color and quality of eggs from hens fed a wheat-based diet. Iran. J. Appl. Anim. Sci. 2015, 5, 167–172. [Google Scholar]
- Rosas-Castor, J.M.; Garza-González, M.T.; García-Reyes, R.B.; Soto-Regalado, E.; Cerino-Córdova, F.J.; García-González, A.; Loredo-Medrano, J.A. Methylene blue biosorption by pericarp of corn, alfalfa, and agave bagasse wastes. Environ. Technol. 2013, 35, 1077–1090. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins. J. Sci. Food Agric. 2018, 99, 1623–1634. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2013, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.-M.; Calado, T.; Guimarães, A.; Rodrigues, M.A.M.; Abrunhosa, L. In vitro adsorption of aflatoxin B1, ochratoxin A, and zearalenone by micronized grape stems and olive pomace in buffer solutions. Mycotoxin Res. 2019, 35, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Perali, C.; Magnoli, A.P.; Aronovich, M.; Rosa, C.A.D.R.; Cavaglieri, L.R. Lithothamnium calcareum (Pallas) Areschoug seaweed adsorbs aflatoxin B1 in vitro and improves broiler chicken’s performance. Mycotoxin Res. 2020, 36, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’ascanio, V.; Visessanguan, W.; Avantaggiato, G. The Effectiveness of Durian Peel as a Multi-Mycotoxin Adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Durán, A.; Nava-Ramírez, M.d.J.; Hernández-Patlán, D.; Solís-Cruz, B.; Hernández-Gómez, V.; Téllez-Isaías, G.; Méndez-Albores, A. Potential of Kale and Lettuce Residues as Natural Adsorbents of the Carcinogen Aflatoxin B1 in a Dynamic Gastrointestinal Tract-Simulated Model. Toxins 2021, 13, 771. [Google Scholar] [CrossRef]
- Maguey-González, J.A.; Nava-Ramírez, M.d.J.; Gómez-Rosales, S.; de Lourdes Angeles, M.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Guzmán, R.; Hernández-Velasco, X.; Figueroa-Cárdenas, J.d.D.; Vázquez-Durán, A.; et al. Humic Acids Preparation, Characterization, and Their Potential Adsorption Capacity for Aflatoxin B1 in an In Vitro Poultry Digestive Model. Toxins 2023, 15, 83. [Google Scholar] [CrossRef]
- Shar, Z.H.; Fletcher, M.T.; Sumbal, G.A.; Sherazi, S.T.H.; Giles, C.; Bhanger, M.I.; Nizamani, S.M. Banana peel: An effective biosorbent for aflatoxins. Food Addit. Contam. Part A 2016, 33, 849–860. [Google Scholar] [CrossRef]
- Karmanov, A.P.; Kanarsky, A.V.; Kanarskaya, Z.A.; Kocheva, L.S.; Semenov, E.I.; Bogdanovich, N.I.; Belyy, V.A. In vitro adsorption-desorption of aflatoxin B1 on Pepper’s lignins isolated from grassy plants. Int. J. Biol. Macromol. 2019, 144, 111–117. [Google Scholar] [CrossRef]
- De Jesus Nava-Ramírez, M.; Salazar, A.M.; Sordo, M.; López-Coello, C.; Téllez-Isaías, G.; Méndez-Albores, A.; Vázquez-Durán, A. Ability of low contents of biosorbents to bind the food carcinogen aflatoxin B1 in vitro. Food Chem. 2021, 345, 128863. [Google Scholar] [CrossRef]
- Phongamwong, T.; Barrabés, N.; Donphai, W.; Witoon, T.; Rupprechter, G.; Chareonpanich, M. Chlorophyll-modified Au25(SR)18-functionalized TiO2 for photocatalytic degradation of rhodamine B. Appl. Catal. B Environ. 2023, 325, 122336. [Google Scholar] [CrossRef]
- Baraka, A.; Dickson, S.; Gobara, M.; El-Sayyad, G.S.; Zorainy, M.; Awaad, M.I.; Hatem, H.; Kotb, M.M.; Tawfic, A.F. Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chem. Pap. 2017, 71, 2271–2281. [Google Scholar] [CrossRef]
- Hernández-Ramírez, J.O.; Merino-Guzmán, R.; Téllez-Isaías, G.; Vázquez-Durán, A.; Méndez-Albores, A. Mitigation of AFB1-Related Toxic Damage to the Intestinal Epithelium in Broiler Chickens Consumed a Yeast Cell Wall Fraction. Front. Vet. Sci. 2021, 8, 677965. [Google Scholar] [CrossRef]
- Nogueira, W.V.; Moyano, F.J.; García, M.J.A.; Tesser, M.B.; Buffon, J.G. Preliminary assessment of bioaccessibility of aflatoxin B1 in fish. Aquac. Int. 2022, 30, 1315–1325. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pusztahelyi, T.; Pócsi, I. Mycotoxins—Prevention and decontamination by yeasts. J. Basic Microbiol. 2015, 55, 805–818. [Google Scholar] [CrossRef]
- Song, R.; Bai, B.; Jing, D. Hydrothermal synthesis of TiO2-yeast hybrid microspheres with controllable structures and their application for the photocatalytic reduction of Cr (VI). J. Chem. Technol. Biotechnol. 2014, 90, 930–938. [Google Scholar] [CrossRef]
- Chen, H.; Dai, G.; Zhao, J.; Zhong, A.; Wu, J.; Yan, H. Removal of copper(II) ions by a biosorbent—Cinnamomum camphora leaves powder. J. Hazard. Mater. 2010, 177, 228–236. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Du, B.; Wei, Q.; Wang, X.C.; Nguyen, P.D. Characterization of a multi-metal binding biosorbent: Chemical modification and desorption studies. Bioresour. Technol. 2015, 193, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Heux, L.; Sugiyama, J. Polymorphism of Cellulose I Family: Reinvestigation of Cellulose IVI. Biomacromolecules 2004, 5, 1385–1391. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef]
- Bai, Y.-P.; Zhou, H.-M.; Zhu, K.-R.; Li, Q. Effect of thermal processing on the molecular, structural, and antioxidant characteristics of highland barley β-glucan. Carbohydr. Polym. 2021, 271, 118416. [Google Scholar] [CrossRef]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Mičušík, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Foods 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Egbedina, A.O.; Ugwuja, C.G.; Dare, P.A.; Sulaiman, H.D.; Olu-Owolabi, B.I.; Adebowale, K.O. CTAB-activated Carbon from Peanut Husks for the Removal of Antibiotics and Antibiotic-resistant Bacteria from Water. Environ. Process. 2023, 10, 20. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2019, 98, 105306. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S.; Buszewski, B. Solvent Influence on Zeta Potential of Stationary Phase—Mobile Phase Interface. Molecules 2022, 27, 968. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar] [CrossRef]
- Zandomeneghi, M.; Festa, C.; Carbonaro, L.; Galleschi, L.; Lenzi, A.; Calucci, L. Front-Surface Absorbance Spectra of Wheat Flour: Determination of Carotenoids. J. Agric. Food Chem. 2000, 48, 2216–2221. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Ćwintal, M.; Niemczynowicz, A.; Boroń, B.; Matwijczuk, A. Effect of Stress Caused by Electromagnetic Stimulation on the Fluorescence Lifetime of Chlorophylls in Alfalfa Leaves. Pol. J. Environ. Stud. 2019, 28, 3133–3143. [Google Scholar] [CrossRef]
- Agati, G.; Fusi, F.; Mazzinghi, P.; di Paola, M.L. A simple approach to the evaluation of the reabsorption of chlorophyll fluorescence spectra in intact leaves. J. Photochem. Photobiol. B Biol. 1993, 17, 163–171. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, N.; Sillanpää, M.; Makgwane, P.R.; Kumar, S.; Kumari, K. Carbon nano-structures and functionalized associates: Adsorptive detoxification of organic and inorganic water pollutants. Inorg. Chem. Commun. 2022, 141, 109579. [Google Scholar] [CrossRef]
- Horwitz, W. Official methods of analysis of AOAC International. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Méndez-Albores, A.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez-Isaías, G.; López-Arellano, R. Comparison of PrestoBlue® and plating method to evaluate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gastrointestinal model. J. Appl. Microbiol. 2018, 124, 423–430. [Google Scholar] [CrossRef]










| Band | Wavenumber (cm−1) | Functional Group | |
|---|---|---|---|
| Alfalfa | YCW | ||
| A | 3668 | NF | O–H stretching |
| B | 3280 | 3281 | O–H and N–H stretching vibrations (carbohydrate and protein) |
| C | 2964 | NF | CH2 antisymmetric stretching (lipids) |
| D | 2917 | 2923 | –(CH2)n– antisymmetric stretching (lipids) |
| E | 2850 | 2853 | C–CH3 symmetric stretching (lipids) |
| F | 1732 | 1710 | C=O stretching (phospholipid esters) |
| G | NF | 1629 | Amide I (N–H bending and C=O stretching) |
| H | 1599 | NF | COOR (carboxylate group) |
| I | NF | 1532 | Amide II (C–N stretching and N–H bending) |
| J | NF | 1455 | OH bending vibration in carboxylic acids |
| K | 1408 | NF | –CH2 deformation (cellulose) |
| L | NF | 1369 | β-anomeric carbons (β-glucans) |
| M | 1242 | 1244 | PO2− antisymmetric stretching (DNA, RNA, phospholipid, phosphorylated protein) |
| N | 1066 | NF | C–O stretching (carbohydrate)jialiC–O–P stretching (phosphate ester) |
| O | NF | 1025 | C–O stretching (carbohydrates) |
| P | NF | 887 | β-anomeric carbons β (1→3)-glucans |
| Q | NF | 812 | Mannans (C–O–C, C–C, and C–OH stretching of pyranose ring) |
| R | NF | 670 | Polysaccharides (α- and β-glucans, α-mannan) |
| S | 611 | NF | NH2 wag (primary amines) |
| T | NF | 575 | Polysaccharides (α- and β-glucans, α-mannan) |
| U | 534 | NF | In plane and out-of-plane ring deformations |
| V | NF | 508 | Polysaccharides (α- and β-glucans, α-mannan) |
| Photosynthetic Pigment | Content (µg/g Dry Weight) | |
|---|---|---|
| Alfalfa | YCW | |
| Chlorophyll a | 1251.2.1 ± 84.4 | ND |
| Chlorophyll b | 1508.0 ± 132.8 | ND |
| Total chlorophyll (a + b) | 2759.1 ± 180.2 | ND |
| Total carotenoid (x + c) | 16.3 ± 94.5 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Ramírez, M.d.J.; Vázquez-Durán, A.; Figueroa-Cárdenas, J.d.D.; Hernández-Patlán, D.; Solís-Cruz, B.; Téllez-Isaías, G.; López-Coello, C.; Méndez-Albores, A. Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models. Toxins 2023, 15, 604. https://doi.org/10.3390/toxins15100604
Nava-Ramírez MdJ, Vázquez-Durán A, Figueroa-Cárdenas JdD, Hernández-Patlán D, Solís-Cruz B, Téllez-Isaías G, López-Coello C, Méndez-Albores A. Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models. Toxins. 2023; 15(10):604. https://doi.org/10.3390/toxins15100604
Chicago/Turabian StyleNava-Ramírez, María de Jesús, Alma Vázquez-Durán, Juan de Dios Figueroa-Cárdenas, Daniel Hernández-Patlán, Bruno Solís-Cruz, Guillermo Téllez-Isaías, Carlos López-Coello, and Abraham Méndez-Albores. 2023. "Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models" Toxins 15, no. 10: 604. https://doi.org/10.3390/toxins15100604
APA StyleNava-Ramírez, M. d. J., Vázquez-Durán, A., Figueroa-Cárdenas, J. d. D., Hernández-Patlán, D., Solís-Cruz, B., Téllez-Isaías, G., López-Coello, C., & Méndez-Albores, A. (2023). Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models. Toxins, 15(10), 604. https://doi.org/10.3390/toxins15100604