1. Introduction
Nowadays, the usage of plants and fruits as alternative supplements has become prominent due to their lesser toxicity and fewer side effects. In addition, the intake of fresh fruits has been shown to play a significant role in reducing the risk of developing chronic diseases, which is a fact that is widely recognised. A tropical fruit tree native to Southeast Asia,
Bouea macrophylla, is also known as Gandaria mango or Plum mango. It is grown commercially in the ASEAN regions (Malaysia, Thailand, and Indonesia). These plants were reported to have beneficial effects, including the fruit, leaves, and seeds of the fruit. The leaves are commonly consumed raw as a salad in Malaysia and other parts of Asia, whereas the fruits are typically used in the preparation of jam, drinks, and ‘rojak’ [
1]. It has been demonstrated that this fruit contains high levels of antioxidant compounds such as flavonoids, polyphenols, vitamin C, and other phytochemical constituents [
2]. These mixtures might be answerable for the pharmacological potential of
Bouea macrophylla as an antioxidant [
1], antimicrobial, anticancer [
3], antihyperglycemic, and antiphotoaging [
4] agent. Consumption of
Bouea macrophylla fruit can also increase the β-carotene concentration in the blood [
5]. However, data on this fruit are still limited and the safety efficacy, particularly in toxicological studies, is still under-explored [
5].
Recently, probiotics have drawn the attention of researchers due to their anti-inflammatory, antioxidant, and anti-tumour properties [
6]. Probiotics are living microorganisms, which, when integrated into yoghurt, are speculated to improve the composition of gut microbiota. In light of the fact that probiotics play an important part in maintaining overall body health and there is a pressing need to find a more value-added and long-term application for
Bouea macrophylla, the product was therefore developed to combine the positive effects of probiotics found in yoghurt with the pharmacological properties of
Bouea macrophylla. However, the dosage and safety of consuming natural extracts are not guaranteed, as there is no published data available regarding the toxicity of
Bouea macrophylla [
5], specifically when it is combined with yoghurt. Hence, the purpose of this research is to investigate the acute and subacute oral toxicity of
Bouea macrophylla yoghurt (BMY) in male Sprague Dawley (SD) rats.
3. Discussion
This study was divided into two phases. In the first phase, ethanol-extracted
Boeau macrophylla (
Figure 4) was incorporated into yoghurt and characterised for its nutritional composition, as well as its phytochemical identification. Based on the nutritional analysis conducted in this study, BMY can be classified under low-sugar yoghurt as it only has 2.72 g of sugar per 100 g. This is according to the cut-off point indicated by European Union (EU) regulations, where a product which contains <5 g of sugar per 100 g can be claimed to be low sugar [
7]. Meanwhile, according to the Code of Federal Regulations (CFR) of the Food and Drug Administration of the United States, the composition of fat, protein, and carbohydrate of our formulated BMY meet the standard sets of yoghurt (fat >3.25; protein
4.4; carbohydrate ≥7.5) [
8].
Based on the phytochemical screening of ethanolic
Boeau macrophylla conducted in this study, most of the compounds identified belong to the phenolic and terpenoid groups. Across the compound list, the phenolic group with diverse sub-classes were detected and flavonoids were the dominant sub-classes discovered, with 32 compounds out of 66 compounds of the phenolic group. According to the previous study, flavonoids have long to be known to possess anticancer, antioxidant, anti-inflammatory, and antiviral properties [
9,
10]. Apart from flavonoids, tannins, and phenolic acid are other groups of sub-classes of the phenolic group that are primarily found in ethanolic
Boeau macrophylla. Both tannins and phenolic acid have been reported to have great antioxidant properties [
11]. Specifically, gallic acid, which is common phenolic acid found in this study, was reported to exhibit a nephroprotective effect [
12]. In this study, the terpenoid group of compounds is also abundant in ethanolic
Boeau macrophylla, which possesses various health benefits. For instance, the previous study demonstrated that terpenoids or isoprenoid display antidiabetic action in mice [
13] and also exhibit cytotoxicity against tumour cells [
14].
In the second part of this study project, the formulated BMY was put through its paces in terms of toxicity testing, which included testing for both acute and subacute oral toxicity. BMY can be considered nontoxic based on the acute toxicity classification method. The LD50 of BMY is greater than 2000 mg/kg of body weight, and a single dose of 2000 mg/kg per body weight did not result in any mortality, behavioural abnormalities, postural changes, or significant differences in the rats’ body weights. As shown in the haematology result (
Table 6), the WBC was reduced in the 2000 mg/kg but was not significantly different to the control, which might be due to the suppressive effect of some components such as flavonoids and tannins in BMY on bone marrow [
15]. There were no perceptible differences found in the relative weight of the organs or the biochemistry of the serum. This was supported by the findings of the histopathological section, which revealed that the kidney, liver, and heart all displayed normal histological characteristics.
Subacute studies provide information on dosage regimens, toxicity to vital organs, and adverse effects that may affect the average life span of an experimental animal. Rats in this study that were subjected to subacute oral toxicity for a period of 28 days showed no signs of mortality, and there were no noticeable changes in their behaviour, regardless of the different doses that were administered (50 mg, 250 mg, 500 mg, and 1000 mg). One of the crucial indications in evaluating an animal’s health status is body weight [
16]. In this study, all rats exhibited normal increments of body weight without any significant differences detected. This indicates that BMY did not interfere with the normal metabolism of the experimented rats [
17]. The findings of this study also indicate that there were no organ injuries either atrophy or hypertrophy or swell [
18,
19], since there were no significant differences in relative organ weight between rats supplemented with various doses of BMY and the subacute control group. This indication could be supported by a histopathological evaluation of vital organs that are influenced by metabolic reactions caused by toxicants [
20]. Through these histopathological evaluation results, no significant damage was found in the three critical organs (liver, kidney, and heart).
In this present study, haematology parameters were analysed to assess for toxicity in BMY. The haematopoietic system is one of the organs that is most affected by toxic substances and is a good indicator of how healthy or sick a person or animal is [
21]. Based on our study, all haematology parameters showed no significant changes except for MCH, MCHC, and lymphocytes. However, the values still lie within the normal reference range [
22,
23]. Remarkably, the WBCs (
Table 11) were slightly reduced across BMY-administrated groups, but were not statistically significantly different to the control group (SC), which might be due to the suppressive effect on growth and differentiation factors in the bone marrow by some components such as flavonoids and tannins in BMY [
15]. In satellite, a subacute toxicity study that serves as an adverse event assessment of BMY exposure showed that all haematology parameters did not exert any significant changes between the control group (SSC) and SST 1000. This suggests our formulated BMY did not alter the production of circulating RBC, WBC, or platelets.
In addition to haematology parameters, the utilisation of blood serum or plasma enzymes can function as a diagnostic tool for the detection of organ damage, cell damage, enzyme induction, activation, or inhibition [
15]. It is possible to use some different blood parameters to evaluate the extent of the damage done to tissues, potential target organs, and organ function impairment. Renal parameters such as sodium, potassium, chloride, urea, and creatinine, as well as liver parameters such as total protein, albumin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), are essential in determining whether or not there has been a change in metabolic function involving the kidney and liver [
24]. In this study, the subacute administration of BMY did not cause any damage to the kidney, as evidenced by the fact that renal parameters did not significantly change in either the control group or the various BMY-supplemented groups. These insignificant changes in renal parameters were also presented in both satellite subacute groups (SSC and SST 1000). Notably, the level of potassium (
Table 12), was slightly increased in the control group (SC) compared across the BMY-administered groups. However, this increment was considered toxicologically irrelevant as this change is not significant statistically. On the other hand, in liver parameters, the level of albumin significantly increased in BMY 50 and BMY 500 groups compared to the SC group. Contrary to this, a significant decrease was detected in the level of globulin in BMY 500 and BMY 1000 compared to the SC group. Yet, the values still lie within the normal reference range [
22]. Total protein ALT, AST, and ALP did not exhibit any significant changes across all groups either within subacute groups or within satellite subacute groups. Insignificant changes in ALT, AST, and ALP, as well as creatinine, serve as good indicators of liver and kidney functions [
25].
5. Materials and Methods
5.1. Collection of Bouea macrophylla
Fresh fruits of Bouea macrophylla were obtained from Kelantan, Malaysia and sent for identification to the Institute of Bioscience, Universiti Putra Malaysia. (UPMSK3154117).
5.2. Extraction Bouea macrophylla Fruit
The flesh of the fruits was separated from the seed and peel. Water was removed from flesh of the fruit by using a fruit juice extractor. Approximately 100 g of ground
Bouea macrophylla flesh were soaked in 300 mL of food-grade ethanol with a sonicator at 50 Hz with a 1 h interval for 3 days [
26]. The mixture was then filtered with Whatman’s filter paper No. 1, and the leftover material was re-extracted twice. The filtrates were combined and then evaporated using a Source Turbo (turbo mode) for 1 h, 5K feet, 40 °C, followed by freeze drying, Labconco, Kansas City, MO.
Bouea macrophylla flesh powdered ethanol extract was kept at −20 °C.
5.3. Preparation of Bouea macrophylla Yoghurt
The incorporation of yoghurt with
Bouea macrophylla fruit extract was conducted based on Fidelis et al. 2021 [
27]. In order to make yoghurt, 100 mL of fresh cow’s milk was brought to a temperature of 72 °C for thirty minutes before being lowered to 45 °C. Approximately, 0.06 g (0.06%
w/w) of starter culture
(Streptococcus salivarius subsp. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) were added. The mixture was incubated using a yoghurt maker at 40–45 °C for 7–10 h until pH dropped to 4.5–4.6 [
27]. After that, the yoghurt was stabilised by cooling at 4 °C overnight. 2 g of
Bouea macrophylla flesh powder extraction was added into 100 mL yoghurt to obtain an approximate final concentration of 2000 mg/100 mL (20 mg/mL).
5.4. Characterisation of Bouea Macrophylla Yoghurt
Nutritional analysis was carried out to determine the protein, energy, fat, sugar and carbohydrate content in the formulated
Bouea macrophylla yoghurt (BMY). The protein content of the BMY was measured using the Kjeldahl method [
28]. BMY yoghurt was placed into a Kjeldahl flask and digested with H
2SO
4 and a catalyst. Dilution with H
2O and neutralisation with sodium thiosulfate to obtain a boric acid solution were then carried out. Then, hydrochloric acid was used to titrate the borate anions, which eventually formed nitrogen. The crude protein was calculated by multiplying with the conversion factor of 6.38 using the formula (1) below:
The number of carbohydrates was determined by using phenol sulfuric acid, and the results were read off using a spectrophotometer at an absorbance of 490 nm [
29]. In order to determine the amount of sugar present in the BMY, the refractometric method was applied. The presence of boundary lines in the refraction field was observed and recorded, and the refractive index was identified [
30]. The amount of fat was determined by following the Garber protocol [
31], and the amount of energy was determined by following the procedure outlined in the Malaysian food composition database [
32]. The Formulas (2 and 3) used to calculate energy (kcal) are as follows:
The pH of the prepared yoghurt was determined using a benchtop pH metre. The microbiological analysis was also performed to determine the numbers of
Lactobacillus spp.,
Bifidobacteria spp., lactic acid bacteria, and titratable acidity as lactic acid.
Lactobacillus spp. and
Bifidobacteria spp. were counted using the pour plate technique and serial dilutions in phosphate-buffer saline (1% PBS). After anaerobic incubation at 37 °C for 72 h, plate counts of
Bifidobacteria spp. and
Lactobacillus spp. were performed in Bifidobacterium agar and MRS agar (pH 6.2) containing 1 mg/L sorbitol, respectively. Results were expressed as colony-forming units per mL (CFU/mL) [
33].
5.5. Phytochemical Screening
UHPLC was used to analyse the chemical components (ACQUITY UPLC I-Class system from Water Corp., Milford, MA, United States). Compounds were separated chromatographically using a column manufactured by Waters called ACQUITY UPLC HSS T3 (100 mm × 2.1 mm × 1.8 μm), which was kept at a temperature of 40 °C. Mobile phases A and B were used in a linear binary gradient with water (containing 0.1% formic acid) and acetonitrile (mobile phase B). During the run, the composition of the mobile phase was altered as follows: at 0 min, it contained 1% B; at 0.5 min, it contained 1% B; at 16.00 min, it contained 35% B; at 18.00 min, it contained 100% B; and at 20.00 min, it contained 1% B. The volume of injection was 1 μL, and the flow rate was set to 0.6 mL/min. The UHPLC system was connected to a hybrid mass spectrometer from Waters called a Vion IMS QTOF. This mass spectrometer was fully equipped with a Lock Spray ion source. The ion source was performed in the negative electron-spray ionisation (ESI) mode under the following particular operating conditions: the capillary voltage was 1.50 kV, the reference capillary voltage was 3.00 kV, the source temperature was 120 °C, the desolvation gas temperature was 550 °C, the desolvation gas flow was 800 L/h, and the cone gas flow was 50 L/h. Nitrogen with a purity greater than 99.5% was used for both the desolvation and the cone gas. The data were collected using the high-definition MSE (HDMSE) mode at a scan time resolution of 0.1 s over the range of
m/
z 50 to 1500. Consequently, in the course of the run, two separate scans with distinct collision energies (CE) were alternately acquired: a low-energy (LE) scan with a constant CE of 4 eV, and a high-energy (HE) scan in which the CE was ramped from 10 to 40 eV. Both scans were performed in the same manner. The collision-induced dissociation (CID) gas used was argon with a purity of 99.9999 per cent [
34].
5.6. Experimental Animal
In this particular study, Sprague Dawley rats that had reached the age of five weeks and weighed between 150 and 200 g on average served as the subjects. Acclimatisation of the rats took place over the course of one week at a temperature range of 23–25 °C and a humidity level of 55–60%. The light/dark cycle was 12 h. The procedures that required the use of animals were carried out with the approval of the Animal Care and Use Committee of the Management and Science University (AE-MSU-073, Date of approval: 15th Feb 2017.
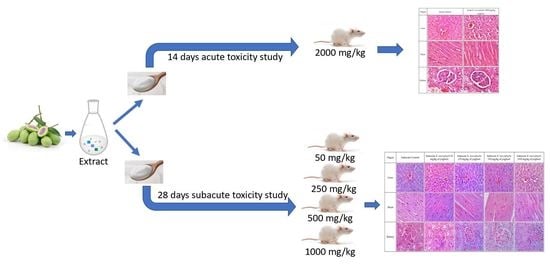
5.7. Acute Toxicity Study
The acute toxicity experiment was performed according to the protocol described in OECD 407 guidelines. Before the acute toxicity experiment, six male Sprague Dawley rats were split into two groups (n = 3). The first group (the controls) received water conveyance. The individuals in Group 2 were given an oral dose of BMY equal to 2000 mg/kg of body weight. After the administration of the substances under study, the rats were monitored at 1, 2, 4, and 6-h intervals for any changes in their general behaviour (including their skin, respiratory activity, tremors, salivation, lethargy, or sleep). Visual observation was carried out to monitor the somatomotor, nervous, circulatory, and respiratory systems. Physical changes, including those to the eyes, fur, skin, and mucous membranes, as well as changes to the behavioural pattern, were also observed. The rats were first observed after 24 h, and then they were observed once a day for the next 14 days. On days 1, 7, and 14, body weight was measured. On day 15, all rats fasted overnight before being anaesthetised and sacrificed. Studies were conducted to determine the LD50 or median lethal dose. Blood and organs were taken for collection.
5.8. Subacute Toxicity Study
A subacute toxicity experiment was performed according to the protocol described in OECD 407 guidelines. In this study, forty-two male rats in good health were used. A normal treatment group and a satellite treatment group comprised the two divisions of this study. In the normal treatment group, rats were put into five groups at random, with six rats in each group (
n = 6). The groups were designated as subacute control (SC), BMY 50 (50 mg/kg), BMY 250 (250 mg/kg), BMY 500 (500 mg/kg), and BMY 1000 (1000 mg/kg), and were fed BMY orally for 28 days. While the rats were in the satellite treatment group, they were put into two treatment groups at random, with six rats in each group (
n = 6). The two groups were called SSC (Satellite Control) and SST (1000 mg/kg), respectively.
Scheme 1 below illustrates the experimental group of animals. The rats were given supplements by mouth for 28 days (for both normal treatment and satellite treatment groups), and then they had 14 days to rest and be watched without treatment (satellite treatment group only). For the evaluation of toxicokinetics, the satellite group was utilised to determine the level of drug exposure in animals that results in an adverse event [
35,
36]. The animals were monitored daily for their overall health as well as for any clinical signs of toxicity throughout the duration of the treatments. Variations in body weight were measured on days 0, 7, 14, 21, and 28 of the experiment, respectively. After the experiment, all of the rats were fasted for a full 24 h before being put under anaesthesia so that the cardiac puncture could be performed to collect blood [
37]. Blood was collected in both EDTA and plain tubes.
5.9. Haematological and Serum Biochemical Analysis
Haematological and biochemical parameters were measured using an automated analyser, Cell Dyn 3700, Abbott Diagnostics, NJ, USA and Cobas C311, Roche Diagnostic, Basel, Switzerland, respectively. For the haematological studies, blood from an EDTA tube was used to measure the haematology parameter, including red blood cell count (RBC), haemoglobin concentration (Hb), haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), platelets (Pt), and white blood cell count (WBC). For the plain tube, the serum was separated for further analysis of plasma aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total proteins, albumin, total and direct bilirubin, urea, creatinine, and ions (sodium, potassium, and chloride) [
37].
5.10. Histopathology Analysis
All of the rats were sacrificed by inhaling carbon dioxide immediately following the cardiac puncture. A surgical procedure was performed to remove samples of organs and tissues of interest, such as the liver, kidneys, and heart. These samples were then weighed and examined macroscopically. After determining the relative organ weight and fixing organ samples in formalin, dehydrating them, and embedding them in paraffin, the results were measured. Subsequently, for histological examination, 5 µm thick sections of each sample were stained with hematoxylin/eosin [
38,
39]. Based on the pathological observations of these tissues, both a macroscopic and a microscopic basis were performed.
5.11. Statistical Analysis
The results for body weight, haematological and serum biochemical parameters, as well as relative organ weight, were all expressed as the mean ± SEM. The data were analysed with a one-way ANOVA, and the Tukey HSD Independent T-test was used to compare the results of the two different groups. Software developed by IBM Corp. in New York, NY, USA called IBM SPSS Statistic version 22.0, was used to carry out the statistical analyses.