β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines
Abstract
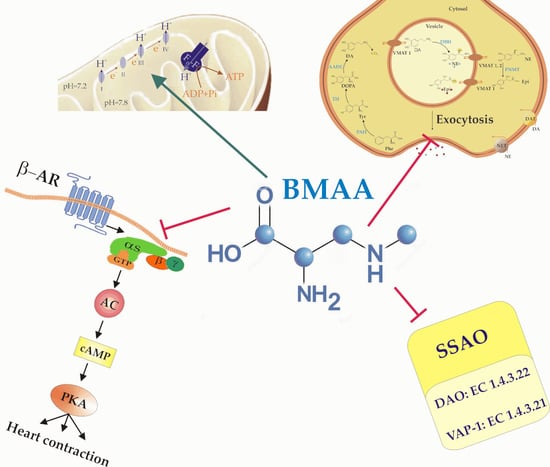
:1. Introduction
2. Results
2.1. Effect of BMAA on ATPase Activity of Intact Mitochondria
2.2. Effect of BMAA on ATPase Activity of Freeze–Thawed Mitochondria and Submitochondrial Particles (SMPs)
2.3. Effect of BMAA on SSAO Activity
2.4. Effect of BMAA on Frog Heart Preparations In Vitro
3. Discussion
3.1. Effect of BMAA on Mitochondrial ATPase
3.2. BMAA Decreases Liver SSAO Activity
3.3. BMAA Toxicity on Sympathetic Neuromediation and Adrenergic Signaling
4. Conclusions
5. Materials and Methods
5.1. Isolation of Intact Rat Liver Mitochondria and SMPs
5.2. Assay of Mitochondrial ATPase Activity
5.3. Assay of Rat Liver SSAO Activity
5.4. Study of Excised Frog Heart Contraction
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ploux, O.; Combes, A.; Eriksson, J.; Metcalf, J.S. β-N-Methylamino-l-Alanine and (S)-2,4-Diaminobutyric Acid Other Cyanobacterial Bioactive Substances. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 182–186. [Google Scholar]
- Gӓrtner, G.; Stoyneva-Gӓrtner, M.; Uzunov, B. Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Jasser, I.; Callieri, C. Monitoring of Cyanobacteria: Sampling Strategies. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: Chichester, UK, 2017; p. 48. [Google Scholar]
- Abbes, S.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Simon, D.F.; Husk, B.; Baulch, H.M.; Vinçon-Leite, B.; Fortin, N.; Greer, C.W.; et al. Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom. Toxins 2022, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.A.; Koksharova, O.A. Neurotoxic Non-proteinogenic Amino Acid β-N-Methylamino-L-alanine and Its Role in Biological Systems. Biochemistry 2016, 81, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, C.; Qiu, J.; Liu, C.; Yan, Y.; Ji, Y.; Wang, G.; Chen, H.; Li, Y.; Li, A. Food web biomagnification of the neurotoxin β-N-methylamino-L-alanine in a diatom-dominated marine ecosystem in China. J. Hazard. Mater. 2021, 404, 124217. [Google Scholar] [CrossRef]
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014, 152, 131–138. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Cui, Y.; Zamyadi, A.; Hofmann, R. Cyanotoxins and Cyanobacteria Cell Accumulations in Drinking Water Treatment Plants with a Low Risk of Bloom Formation at the Source. Toxins 2018, 10, 430. [Google Scholar] [CrossRef]
- Lance, E.; Arnich, N.; Maignien, T.; Biré, R. Occurrence of β-N-methylamino-l-alanine (BMAA) and Isomers in Aquatic Environments and Aquatic Food Sources for Humans. Toxins 2018, 10, 83. [Google Scholar] [CrossRef]
- Lepoutre, A.; Faassen, E.J.; Zweers, A.J.; Lürling, M.; Geffard, A.; Lance, E. How the Neurotoxin β-N-Methylamino-l-Alanine Accumulates in Bivalves: Distribution of the Different Accumulation Fractions among Organs. Toxins 2020, 12, 61. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Pflugmacher, S.; Downing, T.G. The effect of β-N-methylamino-L-alanine (BMAA) on oxidative stress response enzymes of the macrophyte Ceratophyllum demersum. Toxicon 2011, 57, 803–810. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Braidy, N.; Guillemin, G.J.; Welch, J.H.; Neilan, B.A. Gliotoxicity of the cyanotoxin, β-methyl-amino-L-alanine (BMAA). Sci. Rep. 2013, 3, 1482. [Google Scholar] [CrossRef] [Green Version]
- Sazdova, I.; Keremidarska-Markova, M.; Chichova, M.; Uzunov, B.; Nikolaev, G.; Mladenov, M.; Schubert, R.; Stoyneva-Gärtner, M.; Gagov, H.S. Review of Cyanotoxicity Studies Based on Cell Cultures. J. Toxicol. 2022, 2022, 5647178. [Google Scholar] [CrossRef]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust—A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10, 109–117. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-Amino-β-methylaminopropionic Acid (BMAA) Play a Role in Neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef]
- Nunes-Costa, D.; Magalhães, J.D.; G-Fernandes, M.; Cardoso, S.M.; Empadinhas, N. Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef]
- Silva, D.F.; Candeias, E.; Esteves, A.R.; Magalhães, J.D.; Ferreira, I.L.; Nunes-Costa, D.; Rego, A.C.; Empadinhas, N.; Cardoso, S.M. Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J. Neuroinflamm. 2020, 17, 332. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-methylamino-L-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef]
- Rakonczay, Z.; Matsuoka, Y.; Giacobini, E. Effects of L-beta-N-methylamino-L-alanine (L-BMAA) on the cortical cholinergic and glutamatergic systems of the rat. J. Neurosci. Res. 1991, 29, 121–126. [Google Scholar] [CrossRef]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef]
- Jouaville, L.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Cucchiaroni, M.L.; Viscomi, M.T.; Bernardi, G.; Molinari, M.; Guatteo, E.; Mercuri, N.B. Metabotropic glutamate receptor 1 mediates the electrophysiological and toxic actions of the cycad derivative {beta}-N-Methylamino-l-alanine on substantia nigra pars compacta DAergic neurons. J. Neurosci. 2010, 30, 5176–5188. [Google Scholar] [CrossRef] [PubMed]
- Wakhloo, D.; Oberhauser, J.; Madira, A.; Mahajani, S. From cradle to grave: Neurogenesis, neuroregeneration and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neural. Regen. Res. 2022, 17, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.C.; Ashkavand, Z.; Norman, K.R. The Role of Mitochondrial Calcium Homeostasis in Alzheimer’s and Related Diseases. Int. J. Mol. Sci. 2020, 21, 9153. [Google Scholar] [CrossRef]
- Esteves, A.R.; Munoz-Pinto, M.F.; Nunes-Costa, D.; Candeias, E.; Silva, D.F.; Magalhães, J.D.; Pereira-Santos, A.R.; Ferreira, I.L.; Alarico, S.; Tiago, I.; et al. Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria. Gut 2021, 72, 73–89. [Google Scholar] [CrossRef]
- Delcourt, N.; Claudepierre, T.; Maignien, T.; Arnich, N.; Mattei, C. Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy. Toxins 2018, 10, 6. [Google Scholar] [CrossRef]
- Liu, X.; Rush, T.; Zapata, J.; Lobner, D. β-N-methylamino-L-alanine induces oxidative stress and glutamate release through action on system Xc−. Exp. Neurol. 2009, 217, 429–433. [Google Scholar] [CrossRef]
- Van Onselen, R.; Downing, T.G. β-N-methylamino-L-alanine Inhibits Human Catalase Activity: Possible Implications for Neurodegenerative Disease Development. Int. J. Toxicol. 2019, 38, 129–134. [Google Scholar] [CrossRef]
- De Munck, E.; Muñoz-Sáez, E.; Antonio, M.T.; Pineda, J.; Herrera, A.; Miguel, B.G.; Arahuetes, R.M. Effect of β-N-methylamino-L-alanine on oxidative stress of liver and kidney in rat. Environ. Toxicol. Pharmacol. 2013, 35, 193–199. [Google Scholar] [CrossRef]
- Van Onselen, R.; Downing, T.G. BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 2018, 143, 74–80. [Google Scholar] [CrossRef]
- Unzeta, M.; Hernàndez-Guillamon, M.; Sun, P.; Solé, M. SSAO/VAP-1 in Cerebrovascular Disorders: A Potential Therapeutic Target for Stroke and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 3365. [Google Scholar] [CrossRef]
- Andrés, N.; Lizcano, J.M.; Rodríguez, M.J.; Romera, M.; Unzeta, M.; Mahy, N. Tissue activity and cellular localization of human semicarbazide-sensitive amine oxidase. J. Histochem. Cytochem. 2001, 49, 209–217. [Google Scholar] [CrossRef]
- Manasieva, V.; Thakur, S.; Lione, L.A.; Patel, J.; Baydoun, A.; Skamarauskas, J. Semicarbazide-Sensitive Amine Oxidase (SSAO) and Lysyl Oxidase (LOX) Association in Rat Aortic Vascular Smooth Muscle Cells. Biomolecules 2022, 12, 1563. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.N.; Wolf, R.M.; Ralle, M.; Dev, S.; Pierson, H.; Askin, F.; Steele, K.E.; Magnuson, T.H.; Schweitzer, M.A.; et al. Obesity is associated with copper elevation in serum and tissues. Metallomics 2019, 11, 1363–1371. [Google Scholar] [CrossRef]
- Kraemer, M.; Krawczyk, M.; Noor, F.; Grünhage, F.; Lammert, F.; Schneider, J.G. Increased Circulating VAP-1 Levels Are Associated with Liver Fibrosis in Chronic Hepatitis C Infection. J. Clin. Med. 2019, 8, 103. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular Adhesion Protein-1 (VAP-1)/Semicarbazide-Sensitive Amine Oxidase (SSAO): A Potential Therapeutic Target for Atherosclerotic Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 679707. [Google Scholar] [CrossRef]
- Stolen, C.M.; Yegutkin, G.G.; Kurkijärvi, R.; Bono, P.; Alitalo, K.; Jalkanen, S. Origins of Serum Semicarbazide-Sensitive Amine Oxidase. Circ. Res. 2004, 95, 50–57. [Google Scholar] [CrossRef]
- Ilieva, B.; Chichova, M.; Gagov, H.; Sazdova, I. Role of autonomic nervous system in the inotropic effect of obestatin. Curr. Top. Pharmacol. 2022, 26, 31–37. [Google Scholar]
- Sazdova, I.; Ilieva, B.; Minkov, I.; Schubert, R.; Gagov, H. Obestatin as contractile mediator of excised frog heart. Cen. Eur. J. Biol. 2009, 4, 327–334. [Google Scholar] [CrossRef]
- Kennedy, C.; van Onselen, R.; Downing, T.G. β-N-methylamino-l-alanine is a non-competitive inhibitor of vesicular monoamine transporter 2. Toxicon 2023, 222, 106978. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Safronova, N.A. Non-Proteinogenic Amino Acid beta-N-Methylamino-LAlanine (BMAA): Bioactivity and Ecological Significance. Toxins 2022, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Rydberg, S. Transfer of the Neurotoxin β-N-methylamino-l-alanine (BMAA) in the Agro-Aqua Cycle. Mar. Drugs 2020, 18, 244. [Google Scholar] [CrossRef]
- Chichova, M.; Shkodrova, M.; Vasileva, P.; Kirilova, K.; Doncheva-Stoimenova, D. Influence of silver nanoparticles on the activity of rat liver mitochondrial ATPase. J. Nanopart. Res. 2014, 16, 2243. [Google Scholar] [CrossRef]
- Dimitrov, O.; Pavlov, V.; Jotova, I. Effects of female sex hormones on polyamine-oxidizing enzyme activities and polyamine concentrations in immature rat uterus and liver. J. Exp. 1996, 52, 795–798. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkodrova, M.; Mishonova, M.; Chichova, M.; Sazdova, I.; Ilieva, B.; Doncheva-Stoimenova, D.; Raikova, N.; Keremidarska-Markova, M.; Gagov, H. β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines. Toxins 2023, 15, 141. https://doi.org/10.3390/toxins15020141
Shkodrova M, Mishonova M, Chichova M, Sazdova I, Ilieva B, Doncheva-Stoimenova D, Raikova N, Keremidarska-Markova M, Gagov H. β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines. Toxins. 2023; 15(2):141. https://doi.org/10.3390/toxins15020141
Chicago/Turabian StyleShkodrova, Milena, Milena Mishonova, Mariela Chichova, Iliyana Sazdova, Bilyana Ilieva, Dilyana Doncheva-Stoimenova, Neli Raikova, Milena Keremidarska-Markova, and Hristo Gagov. 2023. "β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines" Toxins 15, no. 2: 141. https://doi.org/10.3390/toxins15020141
APA StyleShkodrova, M., Mishonova, M., Chichova, M., Sazdova, I., Ilieva, B., Doncheva-Stoimenova, D., Raikova, N., Keremidarska-Markova, M., & Gagov, H. (2023). β-N-Methylamino-L-Alanine (BMAA) Modulates the Sympathetic Regulation and Homeostasis of Polyamines. Toxins, 15(2), 141. https://doi.org/10.3390/toxins15020141