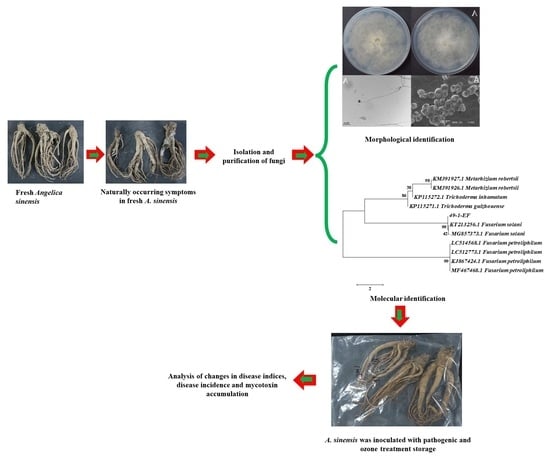
Isolation of the Main Pathogens Causing Postharvest Disease in Fresh Angelica sinensis during Different Storage Stages and Impacts of Ozone Treatment on Disease Development and Mycotoxin Production
Abstract
:1. Introduction
2. Results
2.1. Disease Development in Fresh A. sinensis during Different Storage Stages after Harvest
2.2. Isolation of Molds Potentially Causing Postharvest Disease of A. sinensis during Different Storage Stages
2.3. Morphological Identification of Isolates
2.4. Molecular Biology Identification of Isolates
2.5. Pathogenicity Testing
2.6. Effect of Ozone Treatment on Postharvest Diseases of Fresh A. sinensis
2.7. Effect of Ozone Treatment on the Mycotoxin Accumulation in the Lesion Tissue of A. sinensis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation of Mold Pathogens
5.2. Identification of Molds Potentially Causing Postharvest Disease during Different Storage Stages of Fresh A. sinensis
5.2.1. Morphological Identification of Isolated Molds
5.2.2. Molecular Biological Identification of Isolated Molds
Genomic DNA Extraction
PCR Amplification and Sequencing
5.3. Pathogenicity Testing of Isolates
5.4. Effect of Ozone Treatment on Postharvest Disease of Fresh A. sinensis
5.5. Effect of Ozone Treatment on the Mycotoxin Accumulation in the Lesion Tissue of A. sinensis
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.P.; Guo, Z.B.; Jin, L.; Li, Y.D. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. China J. Nat. Med. 2015, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Lv, J.L.; Chen, H.L.; Duan, J.A.; Liu, J.W. Research progress of structures and pharmacological activities of phthalides from Angelica sinensis. China J. Chin. Mater. Med. 2016, 41, 167–176. [Google Scholar]
- Cao, P.; Sun, J.L.; Sullivan, M.A.; Huang, X.; Wang, H.X.; Zhang, Y.; Wang, N.; Wang, K.P. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.T.; Liu, W.Z.; Niu, T.H.; Du, L.D.; Wang, R.Q.; Ren, Y.; Guo, M. Protective effects of Angelica sinensis volatile oil on atherosclerosis in hyperlipidemia mice. J. Chin. Med. Mater. 2016, 39, 2102–2107. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, L.; Zhu, T.T.; Zeng, C.Y.; Zhang, Y.Y. First report of Angelica sinensis leaf spot caused by Septoria anthrisci in Gansu province, China. Plant Dis. 2018, 102, 442. [Google Scholar] [CrossRef]
- Ma, H.X.; Duan, X.M.; Xu, W.H.; Ma, G.H.; Ma, W.L.; Qi, H.X. Root rot of Angelica sinensis caused by Clonostachys rosea and Fusarium acuminatum in China. Plant Dis. 2022, 106, 2264. [Google Scholar] [CrossRef]
- Bian, J.; Chen, T.X.; Chen, X.R.; Wang, H.Q.; Yang, X.L.; Wang, Y. Pathogen identification and occurrence regularity of a novel disease-Angelica anthracnose. Acta Prataculturae Sin. 2014, 23, 266–273. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhang, J.Q.; Zhang, W.X.; Kou, Z.A.; Wang, X.F.; Liu, L.; Li, Z.Y.; Wang, Y.L.; Shen, T.; Tian, Y.Q. First report of Fusarium avenaceum causing leaf spot on Angelica sinensis in China. Plant Dis. 2021, 106, 1524. [Google Scholar] [CrossRef]
- Ma, D.S.; Gao, F.F.; Li, W.; Gong, X.J.; Zheng, Y.N. Comparative study on adenosine and L-pyroglutamic acid content of fresh ginseng and its processed products. J. Jilin Agric. Univ. 2013, 35, 36–39, 45. [Google Scholar] [CrossRef]
- Yang, C.J.; Wang, L.X.; Feng, L. Content difference of total alkaloids in fresh and dried portulacae herba. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 88–90. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Q.J.; Sun, K.; Guo, Z.X.; Chi, X.L.; Huang, L.Q. Population characteristics and threatened of wild Angelica sinensis in Gansu province. China J. Chin. Mater. Med. 2019, 44, 2987–2995. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Z.; Ji, L.L.; Chen, C.K.; Dong, C.H.; Wang, C.R. Effects of ozone treatment on the storage quality of post-harvest tomato. Int. J. Food Eng. 2018, 14, 20180012. [Google Scholar] [CrossRef]
- De Santis, D.; Garzoli, S.; Vettraino, A.M. Effect of gaseous ozone treatment on the aroma and clove rot by Fusarium proliferatum during garlic postharvest storage. Heliyon 2021, 7, E06634. [Google Scholar] [CrossRef]
- Yazdi, M.T.; Zarrini, G.; Mohit, E.; Faramarzi, M.A.; Setayesh, N.; Sedighi, N.; Mohseni, F.A. Mucor hiemalis: A new source for uricase production. World J. Microbiol. Biotechnol. 2006, 22, 325–330. [Google Scholar] [CrossRef]
- He, W.; An, T.X.; Yu, L. Isolation, purification enzyme activity analysis of domianant fermentation strains from sichuan Taihe Mucor Douchi. J. Anhui Agric. Sci. 2021, 49, 157–161. [Google Scholar] [CrossRef]
- Zhang, B.J. Occurrence regularity and prevention-control measures of Mucor on production of Lentinus edodes. North. Hortic. 2016, 19, 208–209. [Google Scholar]
- Zhang, X.C.; Gao, Z.Y.; Xiao, Q.; Peng, Y.K.; Wang, J.B. Isolation and identification of pathogen fungi species of litchi in China. Guangdong Agric. Sci. 2014, 41, 81–84, 95, 237. [Google Scholar] [CrossRef]
- Jensen, B.; Lübeck, P.; Jørgensen, H.J. Clonostachys rosea reduces spot blotch in barley by inhibiting prepenetration growth and sporulation of Bipolaris sorokiniana without inducing resistance. Pest Manag. Sci. 2016, 72, 2231–2239. [Google Scholar] [CrossRef]
- Yang, R.; Lang, J.F.; Lu, N.H.; Shi, M.W. Inhibition and protective activities of Clonostachys rosea to corn stalk rot. J. Henan Inst. Sci. Technol. Nat. Sci. Ed. 2016, 44, 28–33. [Google Scholar] [CrossRef]
- Li, X.P.; Xu, S.Y.; Li, J.J.; Zhang, Y.X.; Qi, Y.H.; Wang, X.M.; Jiang, J.J.; Fan, Y.X.; Li, M.Q. Clonostachys rosea, a pathogen of root rot in naked barley (Hordeum vulgare L. var. nudum Hook. f.) on the Qinghai-Tibet Plateau, China. Microbiol. China 2022, 49, 598–605. [Google Scholar] [CrossRef]
- Shanawaer, S.; Yushanjiang, M.; Guo, Q.Y.; Bai, J.Y. Identification of the pathogen causing jujube fruit mildew (Part II)—Isolation and identification of Penicillium fungus causing jujube fruit mildew. Xinjiang Agric. Sci. 2016, 53, 698–705. [Google Scholar]
- Puel, O.; Galtier, P.; Oswald, I. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613. [Google Scholar] [CrossRef]
- Mahunu, G.K.; Zhang, H.Y.; Yang, Q.Y.; Li, C.L.; Zheng, X.F. Biological control of patulin by antagonistic yeast: A case study and possible model. Crit. Rev. Microbiol. 2016, 42, 643–655. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zhan, K.; Lu, H.X.; Qi, R.D. Screening and identification of antagonisms against the soilborne pathogens. Chin. Agric. Sci. Bull. 2014, 30, 8–14. [Google Scholar]
- Mincuzzi, A.; Sanzani, S.M.; Palou, L.; Ragni, M.; Ippolito, A. Postharvest rot of pomegranate fruit in southern Italy: Characterization of the main pathogens. J. Fungi 2022, 8, 475. [Google Scholar] [CrossRef]
- Guo, M.Y.; Pang, X.H. Research progress on identification of Aspergillus fungi in traditional Chinese medicinal materials. Chin. Tradit. Herb. Drugs 2018, 49, 3933–3941. [Google Scholar] [CrossRef]
- Zhang, G.H.; Li, D.; He, C.Z. Identification of contaminating endophytes in tissue culture of Annamocarya sinensis. J. Green Sci. Technol. 2022, 24, 169–172. [Google Scholar]
- Viegas, C.; Nurme, J.; Piecková, E.; Viegas, S. Sterigmatocystin in foodstuffs and feed: Aspects to consider. Mycology 2018, 11, 91–104. [Google Scholar] [CrossRef]
- Lu, X.F.; Luo, C.Q.; Xing, J.Y.; Han, Z.Z.; Li, T.; Wu, W.W.; Xu, H.; Zhan, R.T.; Chen, W.W. Optimization of storage conditions of the medicinal herb Ilex asprella against the sterigmatocystin producer Aspergillus versicolor using response surface methodology. Toxins 2018, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.S.; Xu, H.; Wang, W.L.; Zhan, R.T.; Chen, W.W. Simultaneous determination of aflatoxin B1, B2, G1, G2, ochratoxin A, and sterigmatocystin in traditional Chinese medicines by LC–MS–MS. Anal. Bioanal. Chem. 2014, 406, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.A.; Hetmanski, M.T.; Clarke, P.A.; Barnes, K.A.; Startin, J.R. Analytical methods for the determination of sterigmatocystin in cheese, bread and corn products using HPLC with atmospheric pressure ionization mass spectrometric detection. Food Addit. Contam. 1996, 13, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhao, X.X.; Qin, X.M.; Lei, Z.H. Analysis of dominant pathogen community causing Astragalus membranaceus var. mongholicus root rot in Shanxi Province. J. Plant Prot. 2018, 45, 878–885. [Google Scholar] [CrossRef]
- Kanetis, L.; Testempasis, S.; Goulas, V.; Samuel, S.; Myresiotis, C.; Karaoglanidis, G.S. Identification and mycotoxigenic capacity of fungi associated with pre- and postharvest fruit rots of pomegranates in Greece and Cyprus. Int. J. Food Microbiol. 2015, 208, 84–92. [Google Scholar] [CrossRef]
- Wu, X.L.; Cui, G.L.; Liu, F.; Li, L.Y. Isolation and molecular identification of endophytic fungi from Artemisia annua and promoting effect of Trichoderma atroviride on its growth. J. Trop. Subtrop. Bot. 2018, 26, 56–64. [Google Scholar]
- Daryaei, A.; Jones, E.E.; Ghazalibiglar, H.; Glare, T.R.; Falloon, R.E. Culturing conditions affect biological control activity of Trichoderma atroviride against Rhizoctonia solani in ryegrass. J. Appl. Microbiol. 2016, 121, 461–472. [Google Scholar] [CrossRef]
- Kandula, D.R.W.; Jones, E.E.; Stewart, A.; McLean, K.L.; Hampton, J.G. Trichoderma species for biocontrol of soil-borne plant pathogens of pasture species. Biocontrol Sci. Technol. 2015, 25, 1052–1069. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, X.L.; Chen, C.; Li, H.Y. Isolation and identification of the pathogens causing root rot disease of Cymbidium hybrida. J. Henan Agric. Univ. 2007, 41, 85–89. [Google Scholar] [CrossRef]
- Fiby, I.; Sopel, M.M.; Michlmayr, H.; Adam, G.; Berthiller, F. Development and validation of an LC-MS/MS based method for the determination of deoxynivalenol and its modified forms in maize. Toxins 2021, 13, 600. [Google Scholar] [CrossRef]
- Mostafa, A.T.; Doustmorad, Z. Geographic distribution of phylogenetic species of the Fusarium graminearum species complex and their 8-ketotrichothecene chemotypes on wheat spikes in Iran. Mycotoxin Res. 2017, 33, 245–259. [Google Scholar] [CrossRef]
- Yan, P.P.; Liu, Z.Z.; Liu, S.Q.; Yao, L.Y.; Liu, Y.; Wu, Y.N.; Gong, Z.Y. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Wang, Y.; Yu, C.Z.; Gao, J.; Yang, S.Q.; Jia, R.F.; Hu, S.; Zhu, C.X. Isolation and identification of carrot root rot caused by Fusarium solani. J. North. Agric. 2020, 48, 70–75. [Google Scholar] [CrossRef]
- Hu, S.; Gao, J.; Wang, Y.; Wang, Y.; Xi, X.M.; Zhang, J.; Zhou, G.J.; Li, A.L. Isolation and identification of Apium graveolens L. root rot pathogens. J. North. Agric. 2019, 47, 65–69. [Google Scholar] [CrossRef]
- Li, P.F.; Bhattacharjee, P.; Wang, S.C.; Zhang, L.H.; Ahmed, I.; Guo, L.H. Mycoviruses in Fusarium species: An update. Front. Cell. Infect. Microbiol. 2019, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.C.; Lin, Q.; Dong, C.H.; Ji, H.P.; Yu, J.Z.; Chen, C.K.; Zhu, Z.Q.; Ban, Z.J.; Zhang, N.; Bao, Y.Y. Effects of ozone concentration on the postharvest quality and microbial diversity of Muscat Hamburg grapes. RSC Adv. 2020, 10, 9037–9045. [Google Scholar] [CrossRef]
- Chen, C.K.; Zhang, H.J.; Zhang, X.J.; Dong, C.H.; Xue, W.T.; Xu, W.T. The effect of different doses of ozone treatments on the postharvest quality and biodiversity of cantaloupes. Postharvest Biol. Technol. 2020, 163, 111124. [Google Scholar] [CrossRef]
- Zhang, Y.; Mahidul, I.M.M.; Gao, C.C.; Cheng, Y.D.; Guan, J.F. Ozone reduces the fruit decay of postharvest winter jujube by altering the microbial community structure on fruit surface. Microbiol. Res. 2022, 262, 127110. [Google Scholar] [CrossRef]
- Xue, H.L.; Bi, Y.; Hussain, R.; Wang, H.J.; Pu, L.M.; Nan, M.N.; Cheng, X.Y.; Wang, Y.; Li, Y.C. Detection of NEO in muskmelon fruits inoculated with Fusarium sulphureum and its control by postharvest ozone treatment. Food Chem. 2018, 254, 193–200. [Google Scholar] [CrossRef]
- Ong, M.K.; Kazi, F.K.; Forney, C.F.; Ali, A. Effect of gaseous ozone on papaya anthracnose. Food Bioprocess Technol. 2013, 6, 2996–3005. [Google Scholar] [CrossRef]
- Guo, Y.H.; He, L.; Qi, X.; Wang, Y.T.; Guo, S.S.; Yang, C.Q. Effect of ozone on controlling gray mold in grapes. Food Sci. 2017, 38, 273–278. [Google Scholar]
- Wang, L.; Shao, H.L.; Luo, X.H.; Wang, R.; Li, Y.F.; Li, Y.Y.; Luo, Y.P.; Chen, Z.X. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS ONE 2017, 11, e0147613. [Google Scholar] [CrossRef] [PubMed]
- Gibert, S.; Edel-Hermann, V.; Gautheron, E.; Gautheron, N.; Sol, J.M.; Capelle, G.; Galland, R.; Bardon-Debats, A.; Lambert, C.; Steinberg, C. First report of Fusarium avenaceum, Fusarium oxysporum, Fusarium redolens and Fusarium solani causing root rot in pea in France. Plant Dis. 2022, 106, 1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Tang, Y.H.; Qiao, N.; Zhang, D.Z.; Chi, W.J.; Liu, J.; Pan, H.Q.; Li, J.T. First report of colletotrichum black leaf spot on strawberry caused by Colletotrichum Siamense in China. J. Phytopathol. 2022, 170, 279–281. [Google Scholar] [CrossRef]
- Li, L.; Xue, H.L.; Bi, Y.; Zhang, R.; Kouasseu, C.J.; Liu, Q.L.; Nan, M.N.; Pu, L.M.; Prusky, D. Ozone treatment inhibits dry rot development and diacetoxyscirpenol accumulation in inoculated potato tuber by influencing growth of Fusarium sulphureum and ergosterol biosynthesis. Postharvest Biol. Technol. 2022, 185, 111796. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Rhizospheric actinomycetes revealed antifungal and plant-growth-promoting activities under controlled environment. Plants 2022, 11, 1872. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Situ, J.J.; Zhu, Q.F.; Xi, P.G.; Zheng, Y.; Liu, H.X.; Zhou, X.F.; Jiang, Z.D. Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchi. Postharvest Biol. Technol. 2019, 155, 37–46. [Google Scholar] [CrossRef]
- Cao, S.; Yang, N.B.; Zhao, C.; Liu, J.; Han, C.G.; Wu, X.H. Diversity of Fusarium species associated with root rot of sugar beet in China. J. Gen. Plant Pathol. 2018, 84, 321–329. [Google Scholar] [CrossRef]
- Jimdjio, C.K.; Xue, H.L.; Bi, Y.; Nan, M.N.; Li, L.; Zhang, R.; Liu, Q.L.; Pu, L.M. Effect of ambient pH on growth, pathogenicity, and patulin production of Penicillium expansum. Toxins 2021, 13, 550. [Google Scholar] [CrossRef]
- Xue, H.L.; Bi, Y.; Wei, J.M.; Tang, Y.M.; Zhao, Y.; Wang, Y. New method for the simultaneous analysis of types A and B trichothecenes by ultrahigh-performance liquid chromatography coupled with tandem mass spectrometry in potato tubers inoculated with Fusarium sulphureum. J. Agric. Food Chem. 2013, 61, 9333–9338. [Google Scholar] [CrossRef]
- Lepom, P.; Kloss, H. Production of sterigmatocystin by Aspergillus versicolor isolated from roughage. Mycopathologia 1988, 101, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Liu, Y.R.; Tang, Z.S.; Song, Z.X.; Chang, B.J.; Zhang, Y.T.; Liu, C.L. Determination of 10 mycotoxins in Hippophae Fructus medicinal and edible products by ultra-performance liquid chromatography-tandem mass spectrometry. China J. Chin. Mater. Med. 2023, 48, 366–373. [Google Scholar]










| Colony Morphology | Microscopic Morphology | |||||
|---|---|---|---|---|---|---|
| Strain Number | Front Color | Back Color | Texture | Margin | Conidium | Conidiophore |
| 7–1 | grey | beige | cotton wool-like | irregular white | spherical, transparent | few symbiotic branches |
| 7–2 | creamy white | grey | cotton wool-like | irregular white | spherical | sporangium |
| 14–1 | white | light yellow | fluffy | regular white | spherical or near spherical | erect and branch |
| 21–1 | dark green | light yellow | concentric colony | white radial wrinkles | rosette-like bunches, nearly spherical | broom |
| 21–2 | dark green | light yellow | dense felt | irregular white | rosette-like bunches, near-spherical | broom |
| 21–3 | center into green | light yellow | concentric and velvety colony | white radial grooves | spherical | sporangium |
| 28–1 | brown | grey-brown | cotton wool-like | irregular black | stick-like | mostly unbranched |
| 28–2 | dark green | light green | cottony | irregular green | subspherical or ovoid | with short lateral branches |
| 49–1 | light purple | light pink | thin fluffy | white radial | ovate or elliptical | branched or unbranched |
| A. ITS Sequence Identification Results | ||
|---|---|---|
| Strain Number | Evolutionary Branch | Homology |
| 7–1 | Mucor hiemalis | 100% |
| 7–2 | Actinomucor elegans | 93% |
| 14–1 | Clonostachys rosea | 100% |
| 21–1 | Penicillium polonicum | 99% |
| 21–2 | Penicillium crustosum | 100% |
| 21–3 | Aspergillus versicolor | 99% |
| 28–1 | Alternaria alternata | 99% |
| 28–2 | Trichoderma atroviride | 100% |
| 49–1 | Fusarium solani | 100% |
| B. TUB sequence identification results | ||
| Strain Number | Evolutionary Branch | Homology |
| 7–1 | Penicillium polonicum | 99% |
| 7–2 | Penicillium polonicum | 85% |
| 14–1 | Clonostachys rosea | 100% |
| 21–1 | Penicillium polonicum | 85% |
| 21–2 | Penicillium crustosum | 100% |
| 21–3 | Aspergillus versicolor | 100% |
| 28–1 | Alternaria alternata | 99% |
| 28–2 | Trichoderma atroviride | 99% |
| 49–1 | Fusarium solani | 99% |
| C. TEF sequence identification results | ||
| Strain Number | Evolutionary Branch | Homology |
| 49–1 | Fusarium solani | 99% |
| Strain Number | Disease Index | Disease Incidence | ||||
|---|---|---|---|---|---|---|
| Control | Ozone for 1 h | Ozone for 2 h | Control | Ozone for 1 h | Ozone for 2 h | |
| 7–1 | 22.77 ± 2.3 | 14.58 ± 3 | 7.69 ± 2.3 | 78.57 ± 5.1 | 48.33 ± 2.9 | 23.08 ± 2.9 |
| 7–2 | 13.59 ± 1.6 | 6.73 ± 1.9 | 3.0 ± 1.2 | 47.92 ± 1.9 | 26.92 ± 5 | 12.0 ± 7.6 |
| 14–1 | 8.8 ± 2.7 | 4.91 ± 1.6 | 1.34 ± 0.7 | 27.78 ± 5.8 | 17.86 ± 1.8 | 3.45 ± 0.8 |
| 21–1 | 23.44 ± 1.5 | 19.02 ± 3 | 7.69 ± 1.5 | 87.5 ± 8.1 | 63.04 ± 1.3 | 28.85 ± 1.3 |
| 21–2 | 10.42 ± 2.3 | 9.38 ± 1.9 | 2.5 ± 0.9 | 41.67 ± 4.8 | 37.5 ± 4.1 | 10.0 ± 1.6 |
| 21–3 | 66.67 ± 2.1 | 45.83 ± 1.7 | 21.88 ± 2.1 | 87.5 ± 3.6 | 48.89 ± 3.3 | 22.0 ± 1.3 |
| 28–1 | 17.31 ± 1.8 | 9.9 ± 1.4 | 2.17 ± 0.8 | 63.46 ± 2.8 | 37.5 ± 1.3 | 8.7 ± 1.3 |
| 28–2 | 9.89 ± 1.9 | 4.92 ± 1.9 | 2.6 ± 1.04 | 27.39 ± 1.6 | 14.17 ± 2.7 | 7.69 ± 1.5 |
| 49–1 | 20.42 ± 2.3 | 13.59 ± 2.8 | 7.29 ± 1.4 | 74.14 ± 5.7 | 47.83 ± 4.1 | 20.83 ± 1.5 |
| Molds Species | Accumulation of Mycotoxins in Fresh A. sinensis Rotting Tissue | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAT (μg/kg) | 15-ADON (μg/kg) | ST (μg/kg) | |||||||
| Control | Ozone for 1 h | Ozone for 2 h | Control | Ozone for 1 h | Ozone for 2 h | Control | Ozone for 1 h | Ozone for 2 h | |
| P. polonicum | 6166 ± 198.7 | 5417 ± 106.4 | 3166 ± 97.3 | nd | nd | ||||
| F. solani | nd | 117 ± 3.7 | 84 ± 2.6 | 56 ± 3.6 | nd | ||||
| A. versicolor | nd | nd | 101 ± 3.4 | 76 ± 2.3 | 35 ± 2.6 | ||||
| Disease Rating | Symptom |
|---|---|
| 0 | No disease |
| 1 | Fibrous root disease area 0~25% or primary root disease area 0~9% |
| 2 | Fibrous root disease area 25~50% or primary root disease area 10~25% |
| 3 | Fibrous root disease area is greater than 50% or the primary root disease area is 25~50% |
| 4 | Primary root disease area greater than 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Yang, D.; Xue, H.; Liu, Z.; Bi, Y.; Zhang, Y.; Yang, X.; Shang, S. Isolation of the Main Pathogens Causing Postharvest Disease in Fresh Angelica sinensis during Different Storage Stages and Impacts of Ozone Treatment on Disease Development and Mycotoxin Production. Toxins 2023, 15, 154. https://doi.org/10.3390/toxins15020154
Xi J, Yang D, Xue H, Liu Z, Bi Y, Zhang Y, Yang X, Shang S. Isolation of the Main Pathogens Causing Postharvest Disease in Fresh Angelica sinensis during Different Storage Stages and Impacts of Ozone Treatment on Disease Development and Mycotoxin Production. Toxins. 2023; 15(2):154. https://doi.org/10.3390/toxins15020154
Chicago/Turabian StyleXi, Jihui, Dongyun Yang, Huali Xue, Zhiguang Liu, Yang Bi, Yuan Zhang, Xi Yang, and Suqin Shang. 2023. "Isolation of the Main Pathogens Causing Postharvest Disease in Fresh Angelica sinensis during Different Storage Stages and Impacts of Ozone Treatment on Disease Development and Mycotoxin Production" Toxins 15, no. 2: 154. https://doi.org/10.3390/toxins15020154
APA StyleXi, J., Yang, D., Xue, H., Liu, Z., Bi, Y., Zhang, Y., Yang, X., & Shang, S. (2023). Isolation of the Main Pathogens Causing Postharvest Disease in Fresh Angelica sinensis during Different Storage Stages and Impacts of Ozone Treatment on Disease Development and Mycotoxin Production. Toxins, 15(2), 154. https://doi.org/10.3390/toxins15020154