Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions
Abstract
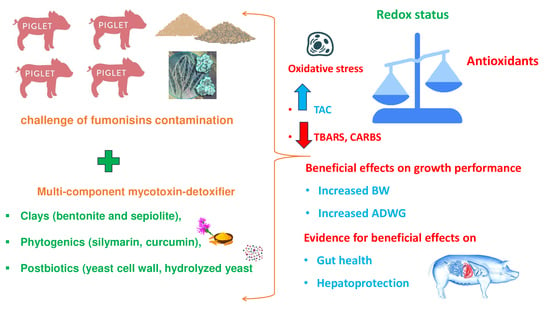
:1. Introduction
2. Results
2.1. Quantification of Mycotoxins in the Feed
2.2. Redox Biomarkers
2.3. Mortality and Performance Parameters
2.4. Gross and Histopathological Lesions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Description of the Farms and Their Diets
5.2. Quantification of Mycotoxins in Feed
5.3. Experimental Material
5.4. Experimental Design
5.4.1. Study 1
5.4.2. Study 2
5.4.3. Conduct of the Studies
5.5. Blood Sampling
5.6. Laboratory Examinations for Redox Biomarkers
5.7. Histopathological Examination
5.8. Mortality and Performance Parameters
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADWG | Average Daily Weight Gain |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFs | Aflatoxins |
| BW | Body Weight |
| CARBs | Protein Carbonyls |
| DNPH | Dinitrophenylhydrazine |
| DON | Deoxynivalenol |
| FCR | Feed Conversion Ratio |
| FI | Total Feed Intake |
| FUM-B1 | Fumonisin B1 |
| FUM-B2 | Fumonisin B2 |
| FUM-B3 | Fumonisin B3 |
| FUM-B4 | Fumonisin B4 |
| FUMs | Fumonisins |
| GIT | Gastrointestinal Tract |
| HPLC-MS | High-Pressure Liquid Chromatography–Mass Spectrometry |
| HT-2 | Trichothecene Toxin HT-2 toxin |
| MDA | Malondialdehyde |
| NRC | National Research Council |
| IPEC | Intestinal Epithelial Cell Line |
| IUGR | Intrauterine Growth Restriction |
| OTA | Ochratoxin A |
| SD | Standard Deviation |
| T-2 | Trichothecene Toxin T-2 toxin |
| TAC | Total Antioxidant Capacity |
| TBARS | Thiobarbituric Acid Reactive Substance |
| TEER | Transepithelial Electrical Resistance |
| ZEN | Zearalenone |
References
- Marroquin-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Gurikar, C.; Shivaprasad, D.P.; Sabillón, L.; Nanje, G.N.A.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. Grain Oil Sci. Technol. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Jedziniak, P. Mycotoxin Biomarkers in Pigs-Current State of Knowledge and Analytics. Toxins 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Bezuidenhout, S.C.; Gelderblom, W.C.; Gorst-Allman, C.P.; Horak, R.M.; Marasas, W.F.; Spiteller, G.; Vleggaar, R.; Gelderblom, W.C.; Jaskiewicz, K.; Marasas, W.F.; et al. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar]
- Rao, Z.X.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Calderón, H.I.; Dritz, S.S. Effects of Fumonisin-Contaminated Corn on Growth Performance of 9 to 28 kg Nursery Pigs. Toxins 2020, 12, 604. [Google Scholar] [CrossRef]
- Yu, S.; Jia, B.; Yang, Y.; Liu, N.; Wu, A. Involvement of PERK-CHOP pathway in fumonisin B1-induced cytotoxicity in human gastric epithelial cells. Food Chem. Toxicol. 2020, 136, 111080. [Google Scholar] [CrossRef]
- Hendel, E.G.; Gott, P.N.; Curry, S.; Hofstetter-Schähs, U.; Murugesan, G.R. Trends in Mycotoxin Contamination in United States Corn. In Proceedings of the Poster Session, Midwest ASAS, Omaha, NE, USA, 2–4 March 2020. [Google Scholar]
- Zomborszky-Kovacs, M.; Vetesi, F.; Horn, P.; Repa, I.; Kovacs, F. Effects of prolonged exposure to low-dose fumonisin B1 in pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 197–201. [Google Scholar] [CrossRef]
- Colvin, B.M.; Cooley, A.J.; Beaver, R.W. Fumonisin toxicosis in swine: Clinical and pathologic findings. J. Vet. Diagn. Investig. 1993, 5, 232–241. [Google Scholar] [CrossRef]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Stylianaki, I.; Tsekouras, N.; Papakonstantinou, G.; Gómez-Nicolau, N.S.; Letsios, M.; Papaioannou, N. Exposure Biomarkers and Histopathological Analysis in Pig Liver After Exposure to Mycotoxins Under Field Conditions: Special Report on Fumonisin B1. Foodborne Pathog. Dis. 2021, 18, 315–321. [Google Scholar] [CrossRef]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarch, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.J.; Van Der Doelen, M.A.M.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81–90. [Google Scholar] [CrossRef]
- Van Le Thanh, B.; Lessard, M.; Chorfi, Y.; dé ric Guay, F. The efficacy of anti-mycotoxin feed additives in preventing the adverse effects of wheat naturally contaminated with Fusarium mycotoxins on performance, intestinal barrier function and nutrient digestibility and retention in weanling pigs. Can. J. Anim. Sci. 2015, 95, 197–209. [Google Scholar] [CrossRef]
- Frobose, H.L.; Stephenson, E.W.; Tokach, M.D.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Goodband, R.D. Effects of potential detoxifying agents on growth performance and deoxynivalenol (DON) urinary balance characteristics of nursery pigs fed DON-contaminated wheat. J. Anim. Sci. 2017, 95, 327–337. [Google Scholar]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for exposure as a tool for efficacy testing of a mycotoxin detoxifier in broiler chickens and pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef]
- Jacela, J.Y.; DeRouchey, J.M.; Tokach, M.D. Feed additives for swine: Fact sheets—Flavors and mold inhibitors, mycotoxin binders, and antioxidants. J. Swine Health Prod. 2010, 18, 27–32. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S. In vivo detoxification of Fusarium toxins. Arch. Anim. Nutr. 2004, 58, 419–441. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Efficacy of Mycotoxin Detoxifiers on Health and Growth of Newly-Weaned Pigs under Chronic Dietary Challenge of Deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.; Kim, D.; Moon, Y. Mycotoxin detoxifiers attenuate deoxynivalenol-induced pro-inflammatory barrier insult in porcine enterocytes as an in vitro evaluation model of feed mycotoxin reduction. Toxicol. Vitr. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Lin, Y.L.; Hsu, Y.C.; Chiu, Y.T.; Huang, Y.T. Antifibrotic effects of a herbal combination regimen on hepatic fibrotic rats. Phytother. Res. 2008, 22, 69–76. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, S.M.; Yen, G.C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox. Signal. 2011, 14, 353–366. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar]
- Williams, J. The Effects of Hops (Humulus lupulus L.) and Silymarin on Performance and Health of Newly Weaned Pigs. Ph.D. Thesis, The Open University, Milton Keynes, UK, September 2007. [Google Scholar]
- Farmer, C.; Lapointe, J.; Cormier, I. Providing the plant extract silymarin to lactating sows: Effects on litter performance and oxidative stress in sows. Animal 2017, 11, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, S.; Lin, Y.; Fang, Z.; Xu, S.; Feng, B.; Zhuo, Y.; Li, J.; Che, L.; Jiang, X.; et al. Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1896–1903. [Google Scholar] [CrossRef]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A. Ethnobotany, phytochemistry and traditional uses of curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): A review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D. Curcumin: New insights into an ancient ingredient against cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Araujo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz. 2011, 96, 723–728. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Yan, E.; Zhang, J.; Han, H.; Wu, J.; Gan, Z.; Wei, C. Curcumin alleviates IUGR jejunum damage by increasing antioxidant capacity through Nrf2/Keap1 pathway in growing pigs. Animals 2019, 10, 41. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z. Dietary curcumin supplementation increases antioxidant capacity, upregulates Nrf2 and hmox1 levels in the liver of piglet model with intrauterine growth retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef]
- Wang, F.; He, J.; Shen, M.; Hang, H.; Niu, Y.; Zhang, L. Effect of curcumin supplementation on intestinal antioxidant function in weaning piglets with intrauterine growth retardation. Food Sci. 2019, 40, 177–183. [Google Scholar]
- Tang, X.; Xiong, K.; Wassie, T.; Wu, X. Curcumin and Intestinal Oxidative Stress of Pigs with Intrauterine Growth Retardation: A Review. Front. Nutr. 2022, 9, 847673. [Google Scholar] [CrossRef]
- Boontiam, W.; Bunchasak, C.; Kim, Y.Y.; Kitipongpysan, S.; Hong, J. Hydrolyzed Yeast Supplementation to Newly Weaned Piglets: Growth Performance, Gut Health, and Microbial Fermentation. Animals 2022, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.; Pistol, G.; Untea, A.; Vlassa, M.; Filip, M.; Anghel, A. Assessment of the ability of dietary yeast-fermented rapeseed meal to modulate inflammatory and oxidative stress in piglets after weaning. J. Anim. Feed Sci. 2022, 31, 109–122. [Google Scholar] [CrossRef]
- Trevisi, P.; Latorre, R.; Priori, D.; Luise, D.; Archetti, I.; Mazzoni, M.; Bosi, P. Effect of feed supplementation with live yeast on the intestinal transcriptome profile of weaning pigs orally challenged with Escherichia coli F4. Animal 2017, 11, 33–44. [Google Scholar] [CrossRef]
- Berto, P.N.; Tse, M.L.P.; Ramos, D.R.A.; Saleh, M.A.D.; Miassi, G.M.; Yamatogi, R.S.; Berto, D.A.; Trindade, N.M.A. Dietary supplementation with hydrolyzed yeast and its effect on the performance, intestinal microbiota, and immune response of weaned piglets. An. Acad. Bras. 2020, 92 (Suppl. S1), e20180969. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 2006, L229, 7–9. [Google Scholar]
- European Commission (EC). Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Union. 2002, 45, 10–22. [Google Scholar]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Matinez-Larranaga, M.R.; Martinez, M.A.; Anadon, A.; Yuan, Z. Fumonisins: Oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of Mycotoxins on Animals’ Oxidative Status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Sun, Y.; Park, I.; Guo, J.; Weaver, A.C.; Kim, S.W. Impacts of low level aflatoxin in feed and the use of modified yeast cell wall extract on growth and health of nursery pigs. Anim. Nutri. 2015, 1, 177–183. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef]
- Celi, P. The role of oxidative stress in small ruminants’ health and production. R. Bras. Zootec. 2010, 39, 348–363. [Google Scholar] [CrossRef]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef]
- Pu, J.; Yuan, Q.; Yan, H.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of Chronic Exposure to Low Levels of Dietary Aflatoxin B1 on Growth Performance, Apparent Total Tract Digestibility and Intestinal Health in Pigs. Animals 2021, 11, 336. [Google Scholar] [CrossRef]
- Ledur, P.C.; Santurio, J.M. Cytoprotective effects of curcumin and silymarin on PK-15 cells exposed to ochratoxin A, fumonisin B1 and deoxynivalenol. Toxicon 2020, 185, 97–103. [Google Scholar] [CrossRef]
- Riahi, I.; Ramos, A.J.; Raj, J.; Jakovčević, Z.; Farkaš, H.; Vasiljević, M.; Pérez-Vendrell, A.M. Effect of a Mycotoxin Binder (MMDA) on the Growth Performance, Blood and Carcass Characteristics of Broilers Fed Ochratoxin A and T-2 Mycotoxin Contaminated Diets. Animals 2021, 11, 3205. [Google Scholar] [CrossRef]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef]
- Holanda, D.M.; Yiannikouris, A.; Kim, S.W. Investigation of the Efficacy of a Postbiotic Yeast Cell Wall-Based Blend on Newly-Weaned Pigs under a Dietary Challenge of Multiple Mycotoxins with Emphasis on Deoxynivalenol. Toxins 2020, 12, 504. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Eliopoulos, C.; Voulgarakis, N.; Arapoglou, D.; Riahi, I.; Sadurní, M.; Papakonstantinou, G.I. Effects of a Multi-Component Mycotoxin-Detoxifying Agent on Oxidative Stress, Health and Performance of Sows. Toxins 2023, 15, 580. [Google Scholar] [CrossRef]
- Farmer, C.; Lapointe, J.; Palin, M.F. Effects of the plant extract silymarin on prolactin concentrations, mammary gland development, and oxidative stress in gestating gilts. J. Anim. Sci. 2014, 92, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ahn, J.M.; Kim, I.H. Micelle silymarin supplementation to sows’ diet from day 109 of gestation to entire lactation period enhances reproductive performance and affects serum hormones and metabolites. J. Anim. Sci. 2021, 99, skab354. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Zhao, Y.; Shen, M.; Zhang, L.; Zhong, X. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Grosu, I.A.; Bulgaru, C.V.; Pistol, G.C.; Cismileanu, A.; Marin, D.E.; Taranu, I. Effects of Exposure to Low Zearalenone Concentrations Close to the EU Recommended Value on Weaned Piglets’ Colon. Toxins 2023, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jang, K.B.; Jalukar, S.; Du, X.; Kim, S.W. Efficacy of Feed Additive Containing Bentonite and Enzymatically Hydrolyzed Yeast on Intestinal Health and Growth of Newly Weaned Pigs under Chronic Dietary Challenges of Fumonisin and Aflatoxin. Toxins 2023, 15, 433. [Google Scholar] [CrossRef]
- Firmin, S.; Gandia, P.; Morgavi, D.P.; Houin, G.; Jouany, J.P.; Bertin, G.; Boudra, H. Modification of aflatoxin B 1 and ochratoxin A toxicokinetics in rats administered a yeast cell wall preparation. Food Addit. Contam. Part A 2010, 27, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.G.; Jeminet, G.; Bertin, G.; Jouany, J.P. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-d-glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Kettunen, H.; Apajalahti, J.; Pennala, E.; Moran, C.A. Comparison of the sequestering properties of yeast cell wall extract and hydrated sodium calcium aluminosilicate in three in vitro models accounting for the animal physiological bioavailability of zearalenone. Food Addit. Contam. Part A 2013, 30, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.C.; See, M.T.; Kim, S.W. Protective effect of two yeast based feed additives on pigs chronically exposed to deoxynivalenol and zearalenone. Toxins 2014, 6, 3336–3353. [Google Scholar] [CrossRef]
- Kim, S.W.; Holanda, D.M.; Gao, X.; Park, I.; Yiannikouris, A. Efficacy of a Yeast Cell Wall Extract to Mitigate the Effect of Naturally Co-Occurring Mycotoxins Contaminating Feed Ingredients Fed to Young Pigs: Impact on Gut Health, Microbiome, and Growth. Toxins 2019, 11, 633. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Szécsi, A.; Szekeres, A.; Bartok, T.; Oros, G.; Bartok, M.; Mesterhazy, M. Fumonisin B1-4 producing capacity of Hungarian Fusarium verticillioides isolates. World Mycotoxin J. 2010, 3, 67–76. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012; p. 399. [Google Scholar]
- Stroka, J.; Anklam, E.; Jorissen, U.; Gilbert, J. Immunoaffinity column cleanup with liquid chromatography using post-column brominatation for determination of aflatoxins in peanut butter, pistachio paste, fig paste, and paprika powder: Collaborative study. J. AOAC Intern. 2000, 83, 320–340. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokkas, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology. Methods in Molecular Biology; Day, C., Ed.; Humana Press: New York, NY, USA, 2014; p. 1180. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 5 November 2023).



| Detected Mycotoxin (µg/kg) | Farm 1 | Farm 2 | Maximum Level (µg/kg) * |
|---|---|---|---|
| Total FUMs | 1220.3 | 2973.0 | (FUM-B1 + FUM-B2) 5000 |
| FUM-B1 | 970.2 | 2309.41 | |
| FUM-B2 | 250.1 | 663.9 | |
| AFB1 | - | 3.9 | 20 |
| AFB2 | <2.0 | <2.0 | |
| AFG1 | <2.0 | <2.0 | |
| AFG2 | <2.0 | <2.0 | |
| OTA | <4.0 | <4.0 | |
| ZEN | <12.0 | <12.0 | |
| DON | <40.0 | <40.0 | |
| T-2 | <4.0 | <4.0 | |
| HT-2 | <40.0 | <40.0 |
| Farm 1 1 | ||||||
|---|---|---|---|---|---|---|
| Parameters | Groups 5 | |||||
| Control (T1) | Experimental (T2) | |||||
| Day 45 | Day 70 | p Value 6 | Day 45 | Day 70 | p Value 6 | |
| TBARS 2 | 9.03 ± 1.09 a | 7.97 ± 0.61 b | <0.001 | 8.48 ± 0.58 g | 6.07 ± 0.42 h | <0.001 |
| CARB 3 | 0.85 ± 0.08 c | 0.75 ± 0.12 d | 0.003 | 0.76 ± 0.09 i | 0.53 ± 0.12 j | 0.027 |
| TAC 4 | 0.51 ± 0.06 e | 0.65 ± 0.10 f | 0.004 | 0.53 ± 0.06 k | 0.70 ± 0.03 l | 0.002 |
| (a,b,c,d,e,f,g,h,i,j,k,l): indicate statistical significance (paired t-test, p ≤ 0.05). | ||||||
| Farm 2 1 | ||||||
| Parameters | Groups 5 | |||||
| Control (T1) | Experimental (T2) | |||||
| Day 45 | Day 70 | p Value 6 | Day 45 | Day 70 | p Value 6 | |
| TBARS 2 | 9.44 ± 0.52 a | 8.51 ± 0.51 b | 0.007 | 8.15 ± 0.56 e | 5.70 ± 0.96 f | 0.002 |
| CARB 3 | 0.90 ± 0.11 c | 0.80 ± 0.06 d | 0.007 | 0.78 ± 0.08 g | 0.63 ± 0.04 h | 0.002 |
| TAC 4 | 0.46 ± 0.02 | 0.51 ± 0.07 | 0.068 | 0.23 ± 0.03 i | 0.59 ± 0.06 j | <0.001 |
| Trial Period | Farm 1 1 | Farm 2 1 | ||||
| Groups 7 | Groups 7 | |||||
| T1 Group | T2 Group | p Value 8 | T1 Group | T2 Group | p Value 8 | |
| Mortality Rate 2 | ||||||
| 8.0 (6/75) | 4.0 (3/75) | <0.001 | 6.66 (5/75) | 2.66 (2/75) | <0.001 | |
| Body Weight (BW) 3 | ||||||
| At weaning age | 7.74 ± 0.68 a | 7.69 ± 0.72 a | 0.870 | 7.51 ± 0.55 a | 7.50 ± 0.63 a | 0.870 |
| Day 45 | 14.86 ± 1.46 a | 17.28 ± 1.54 b | <0.001 | 14.59 ± 1.25 a | 16.88 ± 1.14 b | <0.001 |
| Day 70 | 27.14 ± 1.64 a | 33.72 ± 1.92 b | <0.001 | 26.96 ± 1.36 a | 31.44 ± 1.71 b | <0.001 |
| Average Daily Weight Gain (ADWG) 4 | ||||||
| Days 28–45 | 385.72 ± 21.28 a | 428.55 ± 2.22 b | <0.001 | 378.86 ± 19.98 a | 421.38 ± 1.94 b | <0.001 |
| Days 45–70 | 586.48 ± 22.88 a | 657.83 ± 2.73 b | <0.001 | 580.87 ± 22.49 a | 653.76 ± 2.26 b | <0.001 |
| Feed Intake (FI) 5 | ||||||
| Day 45 | 18.26 ± 1.31 a | 21.6 ± 1.23 c | <0.001 | 18.21 ± 1.68 e | 21.17 ± 1.58 g | <0.001 |
| Day 70 | 40.82 ± 2.13 b | 50.1 ± 2.68 d | <0.001 | 40.44 ± 2.2 f | 47.15 ± 2.62 h | <0.001 |
| Feed Conversion Ratio 6 | ||||||
| Day 45 | 1.25 ± 0.03 a | 1.25 ± 0.03 c | 0.45 | 1.25 ± 0.03 e | 1.25 ± 0.03 g | 0.31 |
| Day 70 | 1.5 ± 0.03 b | 1.5 ± 0.03 d | 0.05 | 1.5 ± 0.03 f | 1.5 ± 0.03 h | 0.95 |
| Composition of Ingredients (kg) | Farm 1 | Farm 2 | ||
|---|---|---|---|---|
| Age of Animals (Days) | ||||
| 28–45 | 45–70 | 28–45 | 45–70 | |
| Corn | 440 | 450 | 506 | 506 |
| Barley | 260 | 200 | 50 | 50 |
| Wheat bran | 40 | 100 | - | - |
| Soybean meal (46% crude protein) | 140 | 145 | 200 | 200 |
| Soybean oil | 20 | 20 | - | - |
| Protein concentrate (68% crude protein) * | 40 | 25 | 40 | 40 |
| Complementary feed with vitamin/mineral premix | - | - | 200 | 200 |
| Supplementary feed with vitamin/mineral premix | 50 | 50 | - | - |
| Mycotoxin binder | 2.5 | 2.5 | 2.5 | 2.5 |
| Tributyrin | - | - | 1.0 | 1.0 |
| Natural calcium carbonate | 7.5 | 7.5 | - | - |
| Total | 1000 | 1000 | 1000 | 1000 |
| Analyzed nutrient components | Age of animals (days) | |||
| 28–45 | 45–70 | 28–45 | 45–70 | |
| Crude protein (%) | 17.10 | 16.40 | 18.0 | 17.5 |
| Crude fat (%) | 4.80 | 4.60 | 3.0 | 3.0 |
| Crude fiber (%) | 3.10 | 3.30 | 3.0 | 3.3 |
| Ash (%) | 4.90 | 4.70 | 5.0 | 5.1 |
| Lysine (%) | 1.17 | 1.11 | 0.9 | 1.3 |
| Methionine + Cystine (%) | 0.72 | 0.70 | 0.62 | 0.60 |
| Calcium (%) | 0.69 | 0.61 | 0.9 | 0.8 |
| Digestible phosphorus (%) | 0.36 | 0.30 | 0.7 | 0.5 |
| Sodium (%) | 0.39 | 0.39 | 0.4 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatsiros, V.G.; Papakonstantinou, G.I.; Voulgarakis, N.; Eliopoulos, C.; Marouda, C.; Meletis, E.; Valasi, I.; Kostoulas, P.; Arapoglou, D.; Riahi, I.; et al. Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions. Toxins 2024, 16, 168. https://doi.org/10.3390/toxins16040168
Papatsiros VG, Papakonstantinou GI, Voulgarakis N, Eliopoulos C, Marouda C, Meletis E, Valasi I, Kostoulas P, Arapoglou D, Riahi I, et al. Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions. Toxins. 2024; 16(4):168. https://doi.org/10.3390/toxins16040168
Chicago/Turabian StylePapatsiros, Vasileios G., Georgios I. Papakonstantinou, Nikolaos Voulgarakis, Christos Eliopoulos, Christina Marouda, Eleftherios Meletis, Irene Valasi, Polychronis Kostoulas, Dimitrios Arapoglou, Insaf Riahi, and et al. 2024. "Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions" Toxins 16, no. 4: 168. https://doi.org/10.3390/toxins16040168
APA StylePapatsiros, V. G., Papakonstantinou, G. I., Voulgarakis, N., Eliopoulos, C., Marouda, C., Meletis, E., Valasi, I., Kostoulas, P., Arapoglou, D., Riahi, I., Christodoulopoulos, G., & Psalla, D. (2024). Effects of a Curcumin/Silymarin/Yeast-Based Mycotoxin Detoxifier on Redox Status and Growth Performance of Weaned Piglets under Field Conditions. Toxins, 16(4), 168. https://doi.org/10.3390/toxins16040168