Calprotectin (S100A8/S100A9) and Myeloperoxidase: Co-Regulators of Formation of Reactive Oxygen Species
Abstract
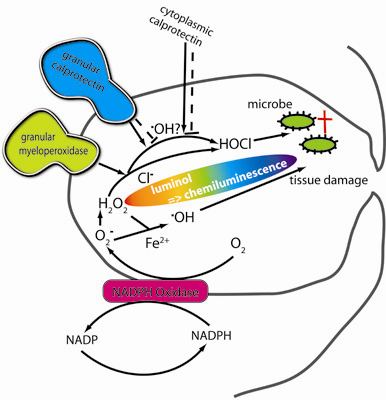
:1. Introduction
2. Results
2.1. Basic response pattern


2.2. The effect of pH changes on MPO- and calprotectin-chemiluminescence, and responses by granulocytes and peritoneal cells

2.3. CL responses of calprotectin and MPO to H2O2, azide, NaCl, melatonin, and cysteine supplements to the reaction mixtures

2.4. The combined effect of calprotectin and NaOCl

2.5. Recombinant S100A8 (A8) and S100A9 (A9)



2.6. 4-hydroxy-benzoic acid (4-HBA) and L-histidine

2.7. Temperature effects
2.8. The effect of iron and iron chelators
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Purification of recombinant S100A8 and S100A9
4.3. Chemiluminescence
4.4. Cell separation
4.5. Statistics
5. Conclusions
Acknowledgements:
References
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [PubMed]
- Kettle, A.J.; Winterbourn, C.C. Superoxide enhances hypochlorous acid production by stimulated human neutrophils. Biochim. Biophys. Acta 1990, 1052, 379–385. [Google Scholar] [PubMed]
- Halliwell, B. Free radicals and oxidative damage in biology and medicine: An introduction. In Oxidative Stress in Skeletal Muscle; Reznick, Z.A., Packer, L., Sen, C.K., Holloszy, J.O., Jackson, M.J., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1998; pp. 1–28. [Google Scholar]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar]
- Tobler, A.; Koeffler, H.P. Myeloperoxidase: Localization, structure, and function. In Blood Cell Biochemistry; Harris, J.R., Ed.; Plenum Press: New York, NY, USA, 1991; Vol. 3, Chapter 10, pp. 255–288. [Google Scholar]
- Fagerhol, M.K.; Dale, I.; Andersson, T. Release and quantitation of a leukocyte derived protein (L1). Scand. J. Haematol. 1980, 24, 393–398. [Google Scholar]
- Johne, B.; Fagerhol, M.K.; Lyberg, T.; Prydz, H.; Brandtzæg, P.; Naess-Andresen, C.F.; Dale, I. Functional and clinical aspects of the myelomonocyte protein calprotectin. J. Clin. Pathol.-Mol. Pathol. 1997, 50, 113–123. [Google Scholar]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003, 60, 569–580. [Google Scholar] [PubMed]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [PubMed]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; Caprioli, R.M.; Nacken, W.; Chazin, W.J.; Skaar, E.P. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [PubMed]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [PubMed]
- Bøyum, A.; Løvhaug, D.; Seeberg, E.; Nordlie, E.M. Identification of cytidine deaminase as inhibitor of granulocyte-macrophage colony formation. Exp. Hematol. 1994, 22, 208–214. [Google Scholar] [PubMed]
- Gran, C.; Bøyum, A.; Johansen, R.F.; Løvhaug, D.; Seeberg, E.C. Growth inhibition of granulocyte-macrophage colony-forming cells by human cytidine deaminase requires the catalytic function of the protein. Blood 1998, 91, 4127–4135. [Google Scholar] [PubMed]
- Bøyum, A.; Rønsen, O.; Tennfjord, V.A.; Tollefsen, S.; Haugen, A.H.; Opstad, P.K.; Bahr, R. Chemiluminescence response of granulocytes from elite athletes during recovery from one or two intense bouts of exercise. Eur. J. Appl. Physiol. 2002, 88, 20–28. [Google Scholar] [PubMed]
- Bøyum, A.; Tennfjord, V.A.; Gran, C.; Løvhaug, D.; Øktedalen, O.; Brandtzæg, P. Bioactive cytidine deaminase,an inhibitor of granulocyte-macrophage colony-forming cells,is massively released in fulminant meningococcal sepsis. J. Infect. Dis. 2000, 182, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Briheim, G.; Stendahl, O.; Dahlgren, C. Intracellular and extracellular events in luminol-dependent chemi-luminescence of polymorphonuclear leukocytes. Infect. Immun. 1984, 45, 1–5. [Google Scholar] [PubMed]
- Yildiz, G.; Demiryürek, A.T.; Sahin-Erdemli, I.; Kanzik, I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Br. J. Pharmacol. 1998, 124, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [PubMed]
- Harrison, C.A.; Raftery, M.J.; Walsh, J.; Alewood, P.; Iismaa, S.E.; Thliveris, S.; Geczy, C.L. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J. Biol. Chem. 1999, 274, 8561–8569. [Google Scholar] [PubMed]
- Test, S.T.; Weiss, S.J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J. Biol. Chem. 1984, 259, 399–405. [Google Scholar] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [PubMed]
- Freinbichler, W.; Bianchi, L.; Colivicchi, M.A.; Ballini, C.; Tipton, K.F.; Linert, W.; Corte, L.D. The detection of hydroxyl radicals in vivo. J. Inorg. Biochem. 2008, 102, 1329–1333. [Google Scholar] [PubMed]
- Ste-Marie, L.; Boismenu, D.; Vachon, L.; Montgomery, J. Evaluation of sodium 4-hydroxybenzoate as an hydroxyl radical trap using gas chromatography-mass spectrometry and high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 1996, 241, 67–74. [Google Scholar] [PubMed]
- Myhre, O.; Vestad, T.A.; Sagstuen, E.; Aarnes, H.; Fonnum, F. The effects of aliphatic (n-nonane), naphtenic (1,2, 4-trimethylcyclohexane), and aromatic (1,2,4-trimethylbenzene) hydrocarbons on respiratory burst in human neutrophil granulocytes. Toxicol. Appl. Pharmacol. 2000, 167, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Khan, A.U.; Karnovsky, M.J. Extracellular production of singlet oxygen by stimulated macrophages quantified using 9,10-diphenylanthracene and perylene in a polystyrene film. J. Biol. Chem. 1993, 268, 15649–15654. [Google Scholar] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [PubMed]
- Dahlgren, C.; Briheim, G. Comparison between the luminol-dependent chemiluminescence of polymorphonuclear leukocytes and of the myeloperoxidase-hooh system - influence of pH, cations and protein. Photochem. Photobiol. 1985, 41, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.; Jungi, T.W. Luminol-enhanced chemiluminescence induced in peripheral blood-derived human phagocytes: Obligatory requirement of myeloperoxidase exocytosis by monocytes. J. Leukoc. Biol. 1993, 54, 300–306. [Google Scholar] [PubMed]
- Gyllenhammar, H. Effects of extracellular pH on neutrophil superoxide anion production, and chemiluminescence augmented with luminol, lucigenin or DMNH. J. Clin. Lab Immunol. 1989, 28, 97–102. [Google Scholar] [PubMed]
- Segal, A.W.; Geisow, M.; Garcia, R.; Harper, A.; Miller, R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 1981, 290, 406–409. [Google Scholar] [PubMed]
- Yousefi, R.; Imani, M.; Ardestani, S.K.; Saboury, A.A.; Gheibi, N.; Ranjbar, B. Human calprotectin: Effect of calcium and zinc on its secondary and tertiary structures, and role of pH in its thermal stability. Acta Biochim. Biophys. Sinica 2007, 39, 795–802. [Google Scholar] [CrossRef]
- Trush, M.A.; Wilson, M.E.; van Dyke, K. The generation of chemiluminescence (CL) by phagocytic cells. In Methods in Enzymology; de Luca, M.A., Ed.; Academic Press, Inc.: New York, NY, USA, 1978. [Google Scholar]
- Brestel, E.P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemi-luminescence. Biochem. Biophys. Res. Commun. 1985, 126, 482–488. [Google Scholar] [PubMed]
- Arnhold, J.; Mueller, S.; Arnold, K.; Grimm, E. Chemiluminescence intensities and spectra of luminol oxidation by sodium hypochlorite in the presence of hydrogen peroxide. J. Biolumin. Chemilumin. 1991, 6, 189–192. [Google Scholar] [PubMed]
- Stendahl, O.; Coble, B.I.; Dahlgren, C.; Hed, J.; Molin, L. Myeloperoxidase modulates the phagocytic-activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J. Clin. Invest. 1984, 73, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Berline, J.; Palefsky, J.M. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J. Leukoc. Biol. 2007, 81, 818–824. [Google Scholar] [PubMed]
- Joshi, S.; Husain, M.M.; Chandra, R.; Hasan, S.K.; Srivastava, R.C. Hydroxyl radical formation resulting from the interaction of nickel complexes of L-histidine, glutathione or L-cysteine and hydrogen peroxide. Hum. Exp. Toxicol. 2005, 24, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and oxidative stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [PubMed]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Raftery, M.J.; Goyette, J.; Hsu, K.; Geczy, C.L. Oxidative modifications of S100 proteins: Functional regulation by redox. J. Leukoc. Biol. 2009, 86, 577–587. [Google Scholar] [PubMed]
- Lim, S.Y.; Raftery, M.; Cai, H.; Hsu, K.; Yan, W.X.; Hseih, H.L.; Watts, R.N.; Richardson, D.; Thomas, S.; Perry, M.; Geczy, C.L. S-nitrosylated S100A8: Novel anti-inflammatory properties. J. Immunol. 2008, 181, 5627–5636. [Google Scholar] [PubMed]
- Korndörfer, I.P.; Brueckner, F.; Skerra, A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 2007, 370, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Hystad, M.E.; Myklebust, J.H.; Bø, T.H.; Sivertsen, E.A.; Rian, E.; Forfang, L.; Munthe, E.; Rosenwald, A.; Chiorazzi, M.; Jonassen, I.; Staudt, L.M.; Smeland, E.B. Characterization of early stages of human B cell development by gene expression profiling. J. Immunol. 2007, 179, 3662–3671. [Google Scholar] [PubMed]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging. Free Rad. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- McCormick, M.M.; Rahimi, F.; Bobryshev, Y.V.; Gaus, K.; Zreiqat, H.; Cai, H.; Lord, R.S.; Geczy, C.L. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J. Biol. Chem. 2005, 280, 41521–41529. [Google Scholar] [PubMed]
- Dale, I.; Fagerhol, M.K.; Naesgaard, I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1-antigen. Eur. J. Biochem. 1983, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teigelkamp, S.; Bhardwaj, R.S.; Roth, J.; Meinardus-Hager, G.; Karas, M.; Sorg, C. Calcium-dependent complex assembly of the myeloic differentiation proteins Mrp-8 and Mrp-14. J. Biol. Chem. 1991, 266, 13462–13467. [Google Scholar] [PubMed]
- Ryckman, C.; Vandal, K.; Rouleau, P.; Talbot, M.; Tessier, P.A. Proinflammatory activities of S100: Proteins S10OA8, S10OA9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003, 170, 3233–3242. [Google Scholar] [PubMed]
- Bøyum, A.; Løvhaug, D.; Tresland, L.; Nordlie, E.M. Separation of leukocytes-improved cell purity by fine adjustments of gradient medium density and osmolality. Scand. J. Immunol. 1991, 34, 697–712. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bøyum, A.; Skrede, K.K.; Myhre, O.; Tennfjord, V.-A.; Neurauter, C.G.; Tolleshaug, H.; Knudsen, E.; Opstad, P.K.; Bjørås, M.; Benestad, H.B. Calprotectin (S100A8/S100A9) and Myeloperoxidase: Co-Regulators of Formation of Reactive Oxygen Species. Toxins 2010, 2, 95-115. https://doi.org/10.3390/toxins2010095
Bøyum A, Skrede KK, Myhre O, Tennfjord V-A, Neurauter CG, Tolleshaug H, Knudsen E, Opstad PK, Bjørås M, Benestad HB. Calprotectin (S100A8/S100A9) and Myeloperoxidase: Co-Regulators of Formation of Reactive Oxygen Species. Toxins. 2010; 2(1):95-115. https://doi.org/10.3390/toxins2010095
Chicago/Turabian StyleBøyum, Arne, Knut Kristian Skrede, Oddvar Myhre, Vivi-Ann Tennfjord, Christine Gran Neurauter, Helge Tolleshaug, Eirunn Knudsen, Per Kristian Opstad, Magnar Bjørås, and Haakon B. Benestad. 2010. "Calprotectin (S100A8/S100A9) and Myeloperoxidase: Co-Regulators of Formation of Reactive Oxygen Species" Toxins 2, no. 1: 95-115. https://doi.org/10.3390/toxins2010095
APA StyleBøyum, A., Skrede, K. K., Myhre, O., Tennfjord, V. -A., Neurauter, C. G., Tolleshaug, H., Knudsen, E., Opstad, P. K., Bjørås, M., & Benestad, H. B. (2010). Calprotectin (S100A8/S100A9) and Myeloperoxidase: Co-Regulators of Formation of Reactive Oxygen Species. Toxins, 2(1), 95-115. https://doi.org/10.3390/toxins2010095