Studies on Carcinogenic and Toxic Effects of Ochratoxin A in Chicks
Abstract
:1. Introduction
2. Materials and Methods
2.1. OTA production
2.2. Experimental design
| Group | Added OTA in feed (ppm - mg/kg) | Added PHE in feed |
|---|---|---|
| Control | none | none |
| 1 | 5 | none |
| 2 | 5 | 0.0025% (0.025 g/kg feed - 25 ppm) L-β PHE |
2.3. Measurements of tumours incidents
2.4. Histological examination
3. Results
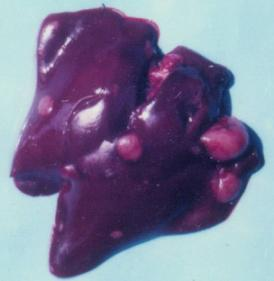
3.1. Clinical observation and gross pathology

| Group | OTA in feed (ppm-mg/kg) | PHE in feed | Kind of neoplasm | Benign or malignant | Time of establishment | Tissues or organs | Sex of chick |
|---|---|---|---|---|---|---|---|
| 1 | 5 | none | adenocarcinoma | malignant | 10th month | liver | male |
| 1 | 5 | none | lymphosarcoma | malignant | 18th month | kidney | male |
| 1 | 5 | none | carcinoma | malignant | 20th month | ureters | male |
| 1 | 5 | none | cystic adenoma | benign | 24th month | kidneys | male |
| 1 | 5 | none | cystic adenoma | benign | 24th month | kidneys | female |
| 2 | 5 | L-b PHE | adenocarcinoma | malignant | 19th month | kidney | female |
| 2 | 5 | L-b PHE | carcinoma | malignant | 21st month | liver, spleen | female |
| 2 | 5 | L-b PHE | rabdomyoma | benign | 24th month | breast muscle | female |



3.2. Histopathology




4. Discussion
5. Conclusions
Acknowledgements
References
- Dwivedi, P.; Burns, R.B. Pathology of ochratoxicosis A in young broiler chicks. Res. Vet. Sci. 1984, 36, 92–103. [Google Scholar]
- Stoev, S.D.; Anguelov, G.; Ivanov, I.; Pavlov, D. Influence of ochratoxin A and an extract of artichoke on the vaccinal immunity and health in broiler chicks. Exp. Toxicol. Pathol. 2000, 52, 43–55. [Google Scholar]
- Stoev, S.D.; Djuvinov, D.; Mirtcheva, T.; Pavlov, D.; Mantle, P. Studies on some feed additives giving partial protection against ochratoxin A toxicity in chicks. Toxicol. Lett. 2002, 135, 33–50. [Google Scholar]
- Haazele, F.M.; Guenter, W.; Marquardt, R.R.; Frohlich, A.A. Benefical effects of dietary ascorbic acid supplement on hens subjected to ochratoxin A toxicosis under normal and high ambient temperatures. Can. J. Anim. Sci. 1993, 73, 149–157. [Google Scholar]
- Stoev, S.D.; Koynarsky, V.; Mantle, P.G. Clinicomorphological studies in chicks fed ochratoxin A while simultaneously developing coccidiosis. Vet. Res. Commun. 2002, 26, 189–204. [Google Scholar]
- Kanisawa, M.; Suzuki, S. Induction of renal and hepatic tumors in mice by ochratoxin A, a mycotoxin. Gann 1978, 69, 599–600. [Google Scholar]
- Castegnaro, M.; Mohr, U.; Pfohl-Leszkowicz, A.; Esteve, J.; Steinmann, J.; Tillmann, T.; Michelon, J.; Bartsch, H. Sex- and strain-specific induction of renal tumors by ochratoxin A in rats correlates with DNA adduction. Int. J. Cancer. 1998, 77, 70–75. [Google Scholar]
- Pfohl-Leszkowicz, A.; Manderville, R. Review on Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar]
- IARC. Ochratoxin A. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans: Some Naturally Occurring Substances; Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, pp. 489–521. [Google Scholar]
- Bunge, I.; Dirheimer, G.; Roschenthaler, R. In vivo and in vitro inhibition of protein synthesis in Bacillus stearothermophilus by ochratoxin A. Biochem. Biophys. Res. Commun. 1978, 83, 398–405. [Google Scholar]
- Creppy, E.E.; Lugnier, A.A.J.; Fasiolo, F.; Heller, K.; Roschenthaler, R.; Dirheimer, G. In vitro inhibition of yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem. Biol. Interact. 1979, 24, 257–261. [Google Scholar]
- Creppy, E.E.; Baudrimont, I.; Betbeder, A.M. Prevention of nephrotoxicity of ochratoxin A, a food contaminant. Toxicol. Lett. 1995, 83, 869–877. [Google Scholar]
- Tapia, M.O.; Seawright, A.A. Experimental ochratoxicosis in pigs. Aust. Vet. J. 1984, 61, 219–222. [Google Scholar]
- Harris, J.P.; Mantle, P.G. The biosynthesis of ochratoxins by Aspergillus ochraceus. Phytochemistry 2001, 58, 709–716. [Google Scholar]
- Gibson, R.M.; Bailey, C.A.; Kubena, L.F.; Huff, W.E.; Harvey, R.B. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 1. Effect on body weight, feed conversion, relative organ weight, and mortality. Poult. Sci. 1990, 69, 414–419. [Google Scholar] [PubMed]
- Bailey, C.A.; Gibson, R.M.; Kubena, L.F.; Huff, W.E.; Harvey, R.B. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 2. Effect on hematology and clinical chemistry. Poult. Sci. 1990, 69, 420–425. [Google Scholar] [PubMed]
- Roth, A.; Chakor, K.; Creppy, E.E.; Kane, A.; Roschenthaler, R.; Dirheimer, G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology 1988, 48, 293–308. [Google Scholar]
- Stoev, S.D.; Daskalov, H.; Radic, B.; Domijan, A.; Peraica, M. Spontaneous mycotoxic nephropathy in Bulgarian chickens with unclarified mycotoxin aetiology. Vet. Res. 2002, 33, 83–94. [Google Scholar]
- Stoev, S.D.; Stefanov, M.; Denev, S.; Radic, B.; Domijan, A.-M.; Peraica, M. Experimental mycotoxicosis in chickens induced by ochratoxin A and penicillic acid and intervention by natural plant extracts. Vet. Res. Commun. 2004, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Doerr, J.A.; Huff, W.E.; Hamilton, B.P.; Lillehoj, E.B. Sever coagulopathy in young chickens produced by ochratoxin A. Toxicol. Appl. Pharm. 1981, 59, 157–163. [Google Scholar]
- Prior, M.C.; Sisodia, C.S. Ochratoxicosis in White Leghorn hens. Poult. Sci. 1978, 57, 619–623. [Google Scholar]
- Munro, I.C.; Moodie, C.A.; Kuiper-Goodman, T.; Scott, P.M.; Grice, H.C. Toxicologic changes in rats fed graded dietary levels of ochratoxin A. Toxicol. Appl. Pharmacol. 1974, 28, 180–188. [Google Scholar]
- Mantle, P.; Kulinskaya, E.; Nestler, S. Renal tumourigenesis in male rats in response to chronic dietary ochratoxin A. Food Addit. Contam. Suppl. 2005, 1, 58–64. [Google Scholar]
- Brown, A.L.; Odell, E.W.; Mantle, P.G. DNA ploidy distribution in renal tumours induced in male rats by dietary ochratoxin A. Exp. Toxicol. Pathol. 2007, 59, 85–95. [Google Scholar]
- NTP. Technical Report on the Toxicology and Carcinogenesis Studies of Ochratoxin A (CAS NO303-47-9) in F344/N Rats (Gavage Studies); NIH Publication No. 89-2813; U.S; U.S. Department of Health and Human Services, National Institutes of Health: Research Triangle Park, NC, USA, 1989.
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Kane, A.; Creppy, E.E.; Dirheimer, G. Differential DNA adduct formation and disappearance in three mice tissues after treatment by the mycotoxin ochratoxin A. Mutation Res. 1993, 289, 265–273. [Google Scholar]
- Manderville, R.; Pfohl-leszkowicz, A. Bioactivation and DNA Adduction as a Rationale for Ochratoxin A Carcinogenesis. World Mycotox. J. 2008, 1, 357–367. [Google Scholar] [CrossRef]
- Bendele, A.M.; Carlton, M.W.; Krogh, P.; Lillehoj, E.B. Ochratoxin A carcinogenesis in the (C57GL/6J x C3H) F1 mouse. J. Natl. Cancer Inst. 1985, 75, 733–742. [Google Scholar]
- Pfohl-Leszkowicz, A.; Pinelli, E.; Bartsch, H.; Mohr, U.; Castegnaro, M. Sex and strain differences in ochratoxin A metabolism and DNA adduction in two strains of rats. Mol. Carcinogen. 1998, 23, 76–83. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Bartsch, H.; Azémar, B.; Mohr, U.; Estève, J.; Castegnaro, M. MESNA protects rats against nephrotoxicity but not carcinogenicity induced by ochratoxin A, implicating two separate pathways. Facta Univ. Ser. Med. Biol. 2002, 9, 57–63. [Google Scholar]
- Pfohl-Leszkowicz, A.; Pinelli, E.; Mohr, U.; Bartsch, H.; Castegnaro, M. Strain and sex specific genotoxic and carcinogenic response of OTA in rats is in part controled by CYP-mediated metabolic reactions. Revue Med. Vet. 1998, 149, 659. [Google Scholar]
- Chernozemsky, I.N.; Stoyanov, I.S.; Petkova–Bocharova, T.K.; Nikolov, I.G.; Draganov, I.V.; Stoichev, I.; Tanchev, Y.; Naidenov, D.; Kalcheva, N.D. Geographic correlation between the occurrence of endemic nephropathy and urinary tract tumours in Vratza district, Bulgaria. J. Cancer 1977, 19, 1–11. [Google Scholar]
- Radovanovic, S.; Jankovic, S.; Jeremovic, J. Incidence of tumours of urinary organs in focus of Balkan endemic nephropathy. Kidney Int. 1991, 40 (Suppl. 34), 75–77. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Castegnaro, M.; Petkova-Bocharova, T.; Nicolov, I.G.; Chernozemsky, I.N.; Bartsch, H.; Betbeder, A.M.; Creppy, E.E.; Dirheimer, G. Ochratoxin A related DNA adducts in urinary tract tumours of Bulgarian subjects. IARC Sci. Publ. 1993, 124, 141–148. [Google Scholar]
- Pfohl-Leszkowicz, A.; Tozlovanu, M.; Manderville, R.; Peraica, M.; Castegnaro, M.; Stefanovic, V. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in Human Nephropathy and Urinary tract tumor. Mol. Nutr. Food Res. 2007, 51, 131–146. [Google Scholar]
- Pfohl-Leszkowicz, A. Ochratoxin A and aristolochic acid in the Nephropathies and associated Urothelial tract Tumours development. Arh. Hig. Rada Toksikol. 2009, 60, 465–483. [Google Scholar]
- Luster, M.I.; Germolec, D.R.; Burleson, G.R.; Jameson, C.W.; Ackermann, M.F.; Lamm, K.R.; Hayes, H.T. Selectiv immunosuppression in mice of natural killer cell activity by ochratoxin A. Cancer Res. 1987, 47, 2259–2263. [Google Scholar]
- Stoev, S.D.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Enedemic Nephropathy. Food Addit. Contam. A 2010, 27, 72–88. [Google Scholar]
- Stoev, S.D.; Denev, S.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A.; Petkov, I. Complex etiology and pathology of mycotoxic nephropathy in South African pigs. Mycotox. Res. 2010, 26, 31–46. [Google Scholar]
- Kanisawa, M. Synergistic effect of citrinin on hepatorenal carcinogenesis of OTA in mice. In Toxigenic fungi - Their Toxins and Health Hazard; Kurata, H., Ueno, Y., Eds.; Kodansha, Tokio and Elsevier: Amsterdam, The Netherlands, 1984; pp. 245–254. [Google Scholar]
- Palmgren, M.S.; Ciegler, A. Toxicity and carcinogenicity of fungal lactones: Patulin and penicillic acid. In Handbook of Natural Toxins; Keeler, R.F., Tu, A.T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1983; pp. 325–341. [Google Scholar]
- Gelderblom, W.C.A.; Marasas, W.F.O.; Farber, E. The cancer initiating potential of the fumonisin Bmycotoxins. Carcinogenesis 1992, 13, 433–437. [Google Scholar]
- Howard, P.C.; Warbritton, A.; Voss, K.A.; Lorenzen, R.J.; Thurman, J.D.; Kovach, R.M.; Bucci, T.J. Compensatory regeneration as a mechanism for renal tubule carcinogenesis of fumonisin B1 in F344/N/Nctr BR rat. Environ. Health Persp. 2001, 109, 309–314. [Google Scholar]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C. An overview of rodent toxicities: Liver and kidney effects of fumonisins and Fusarium moniliforme. Environ. Health Persp. 2001, 109, 259–266. [Google Scholar]
- Bucci, T.J.; Howard, P.C.; Tolleson, W.H.; Laborde, J.B.; Hansen, D.K. Renal effects of fumonisinmycotoxins in animals. Toxicol. Pathol. 1998, 26, 190–194. [Google Scholar]
- Hard, G.C.; Howard, P.C.; Kovatch, R.M.; Bucci, T.J. Rat kidney pathology induced by chronic exposure to fumonisin B1 includes rare variants of renal tubule tumour. Toxicol. Pathol. 2001, 29, 379–386. [Google Scholar]
- Riley, R.T.; Hjinton, D.M.; Chamberlain, W.J.; Bacon, C.W.; Wang, E.; Merrill, A.H.; Voss, K.A. Dietary fumonisin B1 induces disruption of sphingolipid metabolism in Sprague-Dawley rats: A new mechanism of nephrotoxicity. J. Nutr. 1994, 124, 594–603. [Google Scholar]
- Stoev, S.D. Mycotoxic nephropathies in farm animals – diagnostics, risk assessment and prevеntive measures. In Mycotoxins in Farm Animals; Oswald, I., Taranu, I., Pandalai, S.G., Eds.; Transworld Research Network: Kerala, India, 2008; pp. 155–195. [Google Scholar]
- Marquardt, R.R.; Frolich, A.A. A review of recent advances in understanding ochratoxicosis. J. Animal Sci. 1992, 70, 3968–3988. [Google Scholar]
- Creppy, E.E.; Roschenthaler, R.; Dirheimer, G. Inhibition of protein synthesis in mice by ochratoxin A and its prevention by phenylalanine. Food Chem. Toxicol. 1984, 22, 883–886. [Google Scholar]
- Stoev, S.D. Complex etiology, prophylaxis and hygiene control in mycotoxic nephropathies in farm animals and humans. Int. J. Mol. Sci. 2008, 9, 578–605. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stoev, S.D. Studies on Carcinogenic and Toxic Effects of Ochratoxin A in Chicks. Toxins 2010, 2, 649-664. https://doi.org/10.3390/toxins2040649
Stoev SD. Studies on Carcinogenic and Toxic Effects of Ochratoxin A in Chicks. Toxins. 2010; 2(4):649-664. https://doi.org/10.3390/toxins2040649
Chicago/Turabian StyleStoev, Stoycho D. 2010. "Studies on Carcinogenic and Toxic Effects of Ochratoxin A in Chicks" Toxins 2, no. 4: 649-664. https://doi.org/10.3390/toxins2040649
APA StyleStoev, S. D. (2010). Studies on Carcinogenic and Toxic Effects of Ochratoxin A in Chicks. Toxins, 2(4), 649-664. https://doi.org/10.3390/toxins2040649