Effect of Antioxidant Mixtures on Growth and Ochratoxin A Production of Aspergillus Section Nigri Species under Different Water Activity Conditions on Peanut Meal Extract Agar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal strains
2.2. Antioxidants
2.3. Culture medium
2.4. Inoculation and incubation conditions
2.5. Growth parameters
2.6. Ochratoxin A extraction
2.7. Detection and quantification of ochratoxin A
2.8. Statistical analysis
3. Results and Discussion
3.1. Effect of antioxidants treatments on lag phase and growth rate
| Source of variation | Df a | Lag phase | Growth rate | ||
|---|---|---|---|---|---|
| MS b | F c | MS b | F c | ||
| I | 3 | 118472.01 | 10.24* | 47.24 | 15789.44* |
| M | 7 | 17221856.84 | 1493.79* | 399.36 | 99999.99* |
| aw | 2 | 1278196.03 | 108.61* | 40.33 | 13472.66* |
| I × M | 28 | 60021.36 | 5.15* | 31.00 | 10584.69* |
| I × M × aw | 78 | 108999.99 | 9.33* | 10.26 | 3552.52* |
| Strains | aw | Lag phase (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (BHA + PP) (mM) | ||||||||||
| 0 | M 1 | M 2 | M 3 | M 4 | M 5 | M 6 | M 7 | M 8 | ||
| RCP Ga | 0.995 | 11 jk | 21 lmno | 28 fghi | 36 h | 28 fghi | 41 fgh | 68 fg | - | - |
| 0.980 | 20 klm | 30 fghi | 32 ghi | 54 defghi | 40 fgh | 62 ij | 79 g | - | - | |
| 0.930 | 28 fghi | 28 fghi | 57 ij | 118 de | 64 fg | 67 fg | 97 fg | - | - | |
| RCP 203a | 0.995 | 15 klmno | 27 fghi | 22 ijklm | 21 lmno | 45 gh | 60 ij | 86 gh | 117 de | - |
| 0.980 | 18 lmno | 33 fghi | 31 fghi | 40 fgh | 65 fg | 73 defg | 86 gh | - | - | |
| 0.930 | 46 gh | 58 ij | 49 gh | 79 defg | 76 defg | 80 g | 103 fg | - | - | |
| RCP 42b | 0.995 | 10 jk | 26 fghi | 59 ij | 53 defghi | 77 defg | 89 gh | 110 d | - | - |
| 0.980 | 16 klmno | 29 fghi | 32 fghi | 39 fgh | 52 defghi | 93 fg | - | - | - | |
| 0.930 | 27 fghi | 51 defghi | 59 ij | 59 ij | 84 g | 260 a | - | - | - | |
| RCP 191b | 0.995 | 10 jk | 13 ghi | 18 lmno | 26 fghi | 26 fghi | 40 fgh | 45 gh | 216 b | - |
| 0.980 | 21 lmno | 27 fghi | 23 ijklm | 29 fghi | 38 fgh | 34 fghi | 60 ij | - | - | |
| 0.930 | 27 fghi | 47 gh | 42 fgh | 55 defghi | 59 ij | 95 fg | 131 c | - | - | |

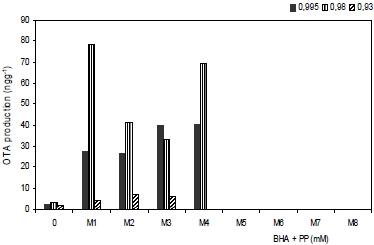
3.2. Effect of antioxidants treatments on ochratoxin A production

| Source of variation | OTA production | |||
|---|---|---|---|---|
| Df a | MS b | F c | Pr > F | |
| I | 3 | 67.65 | 15159.37* | 0.0001 |
| M | 7 | 217.73 | 48788.22* | 0.0001 |
| aw | 2 | 29.66 | 6623.96* | 0.0001 |
| I × M | 28 | 9.33 | 2071.76* | 0.0001 |
| I × M × aw | 78 | 1.45 | 322.30* | 0.0001 |
| Treatments | Growth rate (mm d−1) | Lag phase (h) | OTA production (ng g−1) |
|---|---|---|---|
| Mean ± SD | |||
| Water activity (aw) | |||
| 0.995 | 0.44 ± 0.37 a | 1.93 ± 0.68 a | 1.59 ± 1.37 a |
| 0.980 | 0.41 ± 0.33 a | 1.97 ± 0.74 a | 1.50 ± 1.42 ª |
| 0.930 | 0.28 ± 0.28 b | 2.21 ± 0.76 b | 1.09 ± 1.09 b |
| Antioxidant mixtures BHA + PP (mM) | |||
| M1 (0.5 + 0.5) | 5.72 ± 0.26 a | 1.16 ± 0.55 a | 2.76 ± 2.02 a |
| M2 (1.0 + 0.5) | 3.89 ± 0.16 b | 1.55 ± 0.46 b | 2.64 ± 1.74 a |
| M3 (2.5 + 0.5) | 1.39 ± 0.21 c | 1.88 ± 0.58 c | 1.66 ± 1.47 b |
| M4 (0.5 +1.0) | 1.72 ± 0.75 c | 1.91 ± 0.99 c | 0.45 ± 0.37 c |
| M5 (1.0 +1.0) | 0.87 ± 0.36 d | 2.02 ± 1.00 c | 0.11 ± 0.07 d |
| M6 (2.5 +1.0) | 0.29 ± 0.16 d | 2.36 ± 0.59 d | 0.08 ± 0.02 d |
| M7 (5.0 + 2.5) | 0.17 ± 0.14 d | 2.22 ± 0.49 d | 0.00 ± 0.00 d |
| M8 (10 + 2.5) | 0.00 ± 0.00 d | 2.05 ± 1.00 e | 0.00 ± 0.00 d |
4. Discussion
5. Conclusions
Acknowledgements
References
- Asis, R.; Barrionuevo, D.L.; Giorda, L.M.; Nores, M.L.; Aldao, M.A. Aflatoxin production in six peanut (Arachis hypogaea L.) genotypes infected with Aspergillus flavus and Aspergillus parasiticus, isolated from peanut production areas of Cordoba, Argentina. J. Agric. Food Chem. 2005, 53, 9274–9280. [Google Scholar] [PubMed]
- Bongiovanni, R.M.; Gilleta, M. Asociación Argentina de Economía Agraria. Análisis económico del cultivo de maní bajo diferentes rotaciones y sistemas de labranza. 2009. Available online: http://agro.uncor.edu/~aaea2007/Comunicaciones%20A/Bongiovanni.pdf (Accessed on 15 December 2009).
- Secretaría de Agricultura, Ganadería, Pesca y Alimentación (SAGPyA). 2009. Available online: http://www.sagypa.mecon.gov.ar (Accessed on 15 December 2009).
- Barros, G.; Torres, A.; Palacio, G.; Chulze, S. Aspergillus species from section Flavi isolated from soil at planting and harvest time in peanuts-growing regions of Argentina. J. Sci. Food Agric. 2003, 3, 1303–1307. [Google Scholar]
- Barros, G.; Torres, A.; Chulze, S. Aspergillus flavus population isolated from soil of Argentina's peanut-growing region. Sclerotia production and toxigenic profile. J. Sci. Food Agric. 2005, 85, 2349–2353. [Google Scholar] [CrossRef]
- Magnoli, C.; Astoreca, A.; Ponsone, L.; Chiacchiera, S.; Dalcero, A. Ochratoxin A and the occurrence of ochratoxin A- producing black Aspergilli in stored peanut seeds from Córdoba, Argentina. J. Sci. Food Agric. 2006, 86, 2369–2373. [Google Scholar]
- Magnoli, C.; Astoreca, A.; Ponsone, L.; Fernández-Juri, M.G.; Barberis, C.L.; Dalcero, A.M. Ochratoxin A and Aspergillus section Nigri in peanut seeds at different months of storage in Córdoba, Argentina. Int. J. Food Microbiol. 2007, 119, 213–218. [Google Scholar]
- IARC, Ochratoxin A. In IARC Monographs on the evaluation of carcinogenic risks to human: some naturally occurring substances; food items and constituents, heterocyclic aromatic amines and mycotoxins; International Agency for Research on Cancer: Lyon, France, 1993; 56, pp. 26–32.
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar]
- Magnoli, C.; Astoreca, A.; Ponsone, L.; Combina, M.; Palacio, G.; da Rocha Rosa, C.A.; Dalcero, A.M. Survey of mycoflora and ochratoxin A in dried vine fruits from Argentina markets. Lett. Appl. Microbiol. 2004, 39, 326–331. [Google Scholar]
- Magnoli, C.; Hallak, C.; Astoreca, A.; Ponsone, L.; Chiacchiera, S.; Dalcero, A.M. Occurrence of ochratoxin A- producing fungi in commercial corn kernels in Argentina. Mycopathologia 2006, 161, 53–58. [Google Scholar]
- Ponsone, M.L.; Combina, M.; Dalcero, A.; Chulze, S. Ochratoxin A and ochratoxigenic Aspergillus species in Argentinean wine grapes cultivated under organic and non-organic systems. Int. J. Food Microbiol. 2007, 114, 131–135. [Google Scholar]
- Safety Evaluation of Certain Food Additives. In WHO Food Additives Series: 47, Fifty-sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additive (JECFA); World Health Organization: Geneva, Switzerland, February 2001.
- Rivera-Carriles, K.; Argaiz, A.; Palou, E.; Lopez-Malo, A. Synergistic inhibitory effect of citral with selected phenolics against Zygosaccharomyces bailii. J. Food Prot. 2005, 68, 602–606. [Google Scholar]
- Ahn, Y.J.; Lee, H.S.; Oh, H.S.; Kim, H.T.; Lee, Y.H. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 2005, 46, 2123–2129. [Google Scholar]
- Etcheverry, M.; Torres, A.; Ramirez, M.L.; Chulze, S.; Magan, N. In vitro control of growth and fumonisin production by Fusarium verticilloides and Fusarium proliferatum using antioxidants under different water availability and temperature regimes. J. Appl. Microbiol. 2002, 92, 624–632. [Google Scholar]
- Reynoso, M.; Torres, A.; Ramírez, M.L.; Rodríguez, M.; Chulze, S.; Magan, N. Efficacy of antioxidant mixtures on growth, fumonisins production and hydrolytic enzyme production by Fusarium verticillioides and F. proliferatum in vitro on maize-based media. Mycol. Res. 2002, 106, 1093–1099. [Google Scholar] [CrossRef]
- Torres, A.M.; Ramírez, M.L.; Arroyo, M.; Chulze, S.; Magan, N. Potencial use of antioxidants for control of growth and fumonisin production by Fusarium proliferatum and Fusarium verticilloides on whole maize grain. Int. J. Food Microbiol. 2003, 83, 319–324. [Google Scholar]
- Selvi, A.T.; Joseph, G.S.; Jayaprakasha, G.K. Inhibition of growth and aflatoxin production in Aspergillus flavus by Garcinia indica extract and its antioxidant activity. Food Microbiol. 2003, 20, 455–460. [Google Scholar]
- Nesci, A.; Rodriguez, M.; Etcheverry, M. Control of Aspergillus growth and aflatoxins production using antioxidants at different conditions of water activity and pH. J. Appl. Microbiol. 2003, 95, 279–287. [Google Scholar]
- Joseph, G.S.; Jayaprakasha, G.K.; Selvi, A.T.; Jena, B.S.; Sakariah, K.K. Antiaflatoxigenic and antioxidant activities of Garcinia extracts. Int. J. Food Microbiol. 2005, 101, 153–160. [Google Scholar]
- Farnochi, C.; Torres, A.; Magan, N.; Chulze, S. Effect of antioxidants and competing mycoflora on Fusarium verticilloides and F. proliferatum populations and fumonisin production on maize grain. J. Stored Prod. Res. 2005, 41, 211–219. [Google Scholar] [CrossRef]
- Passone, M.A.; Resnik, S.L.; Etcheverry, M.G. In vitro effect of phenolic antioxidants on germination, growth and aflatoxin B1 accumulation by peanut Aspergillus section Flavi. J. Appl. Microbiol. 2005, 99, 682–691. [Google Scholar]
- Passone, M.A.; Resnik, S.L.; Etcheverry, M.G. Antiaflatoxigenic property of food grade antioxidants under different conditions of water activity in peanut grains. Int. J. Food Microbiol. 2007, 118, 8–14. [Google Scholar]
- Palumbo, J.; O’Keeffe, T.; Mahoney, N. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia 2007, 164, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, F.; Mascoti, L.; Sanchez, C.; Garibotto, F.; Giannini, F.; Kurina-Sanz, M.; Enriz, R. Structure-antifungal activity relationship of cinnamic acid derivatives. J. Agric. Food Chem. 2007, 55, 10635–10640. [Google Scholar]
- Romero, S.M.; Alberto, M.R.; Vaamonde, G. Effect of gallic acid on Aspergillus carbonarius growth and ochratoxin A production. World Mycotox. J. 2010, 3, 45–48. [Google Scholar]
- Barberis, C.; Astoreca, A.; Fernández-Juri, M.G.; Chulze, S.; Magnoli, C.; Dalcero, A. Use of propyl paraben to control growth and ochratoxin A production by Aspergillus section Nigri species on peanut meal extract agar. Int. J. Food Microbiol. 2009, 136, 133–136. [Google Scholar]
- Barberis, C.; Astoreca, A.; Asili, R.; Fernández-Juri, M.G.; Chulze, S.; Magnoli, C.; Dalcero, A. In vitro control of growth and ochratoxin A production by butylatedhydroxyanisole in Aspergillus section Nigri species. Food Contr. 2009, 20, 709–715. [Google Scholar]
- Samson, R.A.; Noonin, P.; Meijer, M.; Houbraken, J.; Frisvad, J.C.; Varga, J. Diagnostic tools to identify black Aspergilli. Stud. Mycol. 2007, 59, 129–145. [Google Scholar]
- Klich, M.A. Identification of common Aspergillus species; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; p. 116. [Google Scholar]
- Marín, S.; Sanchis, V.; Viñas, I.; Canela, R.; Magan, N. Effect of water activity and temperature on growth and fuminisin B1 and B2 production by Fusarium proliferatum and F. moniliforme on maize grain. Lett. Appl. Microbiol. 1995, 21, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [Google Scholar]
- Scudamore, K.A.; MacDonald, S.J. A collaborative study of an HPLC method for determination of ochratoxin A in wheat using immunoaffinity column clean-up. Food Addit. Contam. 1998, 15, 401–410. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; p. 537. [Google Scholar]
- Nesci, A.; Gsponer, N.; Etcheverry, M. Natural maize phenolic acids for control of aflatoxigenic fungi on maize. J. Food Sci. 2007, 72, 180–185. [Google Scholar]
- Smith, J.E.; Moss, M.O. Mycotoxins. Formation, Analyses and Significance; Wiley: Chichester, UK, 1985; pp. 10–31. [Google Scholar]
- Lacey, J. Prevention of mould growth and mycotoxin production through control of environmental factors. In Mycotoxins and Phycotoxins. Bioactive Molecules; Natori, S., Hashimoto, K., Ueno, Y., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1989; Volume 10, pp. 161–169. [Google Scholar]
- Marín, S.; Magan, N.; Albellana, M.; Caenal, R.; Ramos, A.J.; Sanchis, V. Selective effect of propionates and water activity on maize mycoflora and impact on fumonisin B1 accumulation. J. Stored Prod. Res. 2000, 36, 203–214. [Google Scholar]
- Bluma, R.; Amaiden, M.R.; Daghero, J.; Etcheverry, M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J. Appl. Microbiol. 2008, 105, 203–214. [Google Scholar]
- Romero, S.M.; Alberto, M.R.; Manca de Nadra, M.C.; Vaamonde, G. Inhibition of growth and ochratoxin A biosynthesis in Aspergillus carbonarius by flavonoid and nonflavonoid compounds. Mycotox. Res. 2009, 25, 165–170. [Google Scholar]
- Liewen, M.B. Antifungal food additivies. In Handbook of Applied Mycology. Food and Feeds; Arora, D.K., Mukerji, K.G., Marth, E.H., Eds.; Marcell dekker: New York, NY, USA, 1991; Volume 3, pp. 541–552. [Google Scholar]
- Khan, S.H.; Aked, J.; Magan, N. Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathol. 2001, 50, 601–608. [Google Scholar]
- Passone, M.A.; Funes, G.J.; Resnik, S.L.; Etcheverry, M.G. Residue levels of food-grade antioxidants in postharvest treated in-pod peanuts during five months of storage. Food Chem. 2008, 106, 691–697. [Google Scholar]
- Codex alimentarius, Food additive details. Update up to the twenty-ninth session of the codex alimentarius commission; The Joint FAO/WHO Committee on Food Additives. WHO: Geneva, Switzerland, July 2006. Available online: http://www.codexalimentarius.net/web/jecfa (Accessed on 15 April 2010).
- Degré, R.; Sylvestre, M. Effect of butylated hydroxyanisole on the cytoplasmic membrane of Staphylococcus aureus Wood 46. J. Food Protect. 1983, 46, 206–209. [Google Scholar]
- Aldunate, J.; Coloma Torres, L.; Spenser, P.; Morello, A.; Ojeda, J.M.; Repetto, Y. Effects of 2-(3)-tert-butyl-4-hydroxyanisole (BHA) on in situ mitochondria of Tripanosoma cruzi. FEBS Lett. 1992, 303, 73–76. [Google Scholar]
- Eklund, T. Organic acids and esters. In Mechanisms of Action of Food Preservation Procedures; Gould, G.W., Ed.; Elsevier Applied Science: New York, NY, USA, 1989; pp. 181–182. [Google Scholar]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barberis, C.; Astoreca, A.; Fernandez-Juri, M.G.; Dalcero, A.M.; Magnoli, C. Effect of Antioxidant Mixtures on Growth and Ochratoxin A Production of Aspergillus Section Nigri Species under Different Water Activity Conditions on Peanut Meal Extract Agar. Toxins 2010, 2, 1399-1413. https://doi.org/10.3390/toxins2061399
Barberis C, Astoreca A, Fernandez-Juri MG, Dalcero AM, Magnoli C. Effect of Antioxidant Mixtures on Growth and Ochratoxin A Production of Aspergillus Section Nigri Species under Different Water Activity Conditions on Peanut Meal Extract Agar. Toxins. 2010; 2(6):1399-1413. https://doi.org/10.3390/toxins2061399
Chicago/Turabian StyleBarberis, Carla, Andrea Astoreca, María Guillermina Fernandez-Juri, Ana María Dalcero, and Carina Magnoli. 2010. "Effect of Antioxidant Mixtures on Growth and Ochratoxin A Production of Aspergillus Section Nigri Species under Different Water Activity Conditions on Peanut Meal Extract Agar" Toxins 2, no. 6: 1399-1413. https://doi.org/10.3390/toxins2061399
APA StyleBarberis, C., Astoreca, A., Fernandez-Juri, M. G., Dalcero, A. M., & Magnoli, C. (2010). Effect of Antioxidant Mixtures on Growth and Ochratoxin A Production of Aspergillus Section Nigri Species under Different Water Activity Conditions on Peanut Meal Extract Agar. Toxins, 2(6), 1399-1413. https://doi.org/10.3390/toxins2061399