Influence of Botulinum Toxin Therapy on Postural Control and Lower Limb Intersegmental Coordination in Children with Spastic Cerebral Palsy
Abstract
:1. Introduction
2. Results
2.1. Trunk Movements
| Spastic diplegia | Spastic hemiplegia | |||
|---|---|---|---|---|
| n = 24 | Pre-BTX | Post-BTX | Pre-BTX | Post-BTX |
| Trunk sag. (ROM, deg) | 8.9 ± 4.4 | 8.5 ± 4.0 | 10.9 ± 13.2 | 6.2 ± 2.3 |
| Trunk front. (ROM, deg) | 5.6 ± 2.7 | 6.3 ± 3.0 | 6.2 ± 3.5 | 5.9 ± 2.2 |
| Trunk trans. (ROM, deg) | 12.2 ± 4.7 | 15.2 ± 7.7 | 9.1 ± 5.6 | 12.4 ± 4.6 |
| Variance of PV1 (%) | 76.6 ± 4.3 | 72.9 ± 5.3 | 77.8 ± 6.0 | 76.9 ± 4.4 |
| Variance of PV2 (%) | 23.1 ± 4.3 | 26.8 ± 5.3 | 21.6 ± 5.9 | 22.7 ± 4.3 |
| Variance of PV3 (%) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.6 ± 0.4 | 0.5 ± 0.2 |
| Covariation plane angle (deg) | 10.3 ± 4.7 | 8.4 ± 4.1 | 9.6 ± 5.0 | 7.1 ± 4.2 |
| Minimal relative phase | −124.6 ± 13.4 | −124.7 ± 19.7 | −143.2 ± 14.3 | −140.8 ± 8.1 |
| Spastic diplegia | Spastic hemiplegia | |||
|---|---|---|---|---|
| n = 24 | Pre-BTX | Post-BTX | Pre-BTX | Post-BTX |
| ROM Trunk sag (deg) | 12.2 ± 5.6 | 12.3 ± 5.8 | 9.6 ± 4.7 | 8.1 ± 3.0 |
| ROM Trunk front (deg) | 7.4 ± 1.9 | 10.0 ± 3.4 | 5.7 ± 2.0 | 8.3 ± 2.7 |
| ROM Trunk trans (deg) | 22.3 ± 6.0 | 21.4 ± 6.9 | 16.3 ± 4.9 | 17.9 ± 9.7 |
| Variance of PV1 (%) | 79.5 ± 6.2 | 77.3 ± 6.0 | 77.6 ± 5.9 | 78.2 ± 3.1 |
| Variance of PV2 (%) | 20.4 ± 6.3 | 22.4 ± 6.3 | 21.9 ± 6.0 | 21.5 ± 3.1 |
| Variance of PV3 (%) | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.4 ± 0.3 | 0.3 ± 0.2 |
| Covariation plane angle (deg) | 10.2 ± 3.0 | 10.1 ± 4.1 | 7.8 ± 4.7 | 9.7 ± 5.2 |
| Minimal Relative Phase | −131.2 ± 12.0 | −140.9 ± 10.9 | −143.6 ± 9.1 | −146.6 ± 7.5 |
2.2. Lower Limb Coordination


3. Discussion
3.1. Trunk Stability
3.2. Lower Limb Kinematics
4. Experimental Section
4.1. Study Population
Patients
| Pt | Age (year) | Gender | CP type | Gestage (week) | Indep. walking | GFMCS | Cognitive impairment | Epilepsy | Injected muscles | BTX dose (U) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | M | R Hemi | 39 | 16 m | II | None | No | ham, gastr | 11 |
| 2 | 3 | F | R Hemi | 42 | 14 m | I | mild | No | gastr, sol | 8 |
| 3 | 4 | F | R Hemi | 38 | 21 m | II | None | No | ham, gastr | 16.5 |
| 4 | 5 | M | R Hemi | 38 | 14 m | II | None | No | ham, gastr | 12 |
| 5 | 12 | M | R Hemi | 39 | 24 m | I | None | No | ps, add, ham, gastr | 15 |
| 6 | 3 | M | R Hemi | 40 | ? | I | None | No | ham, gastr, sol | 12 |
| 7 | 4 | M | R Hemi | 33 | 21 m | I | Mild | C | add, ham, gastr | 12.5 |
| 8 | 11 | M | R Hemi | 40 | ? | II | None | No | ham, gastr | 12 |
| 9 | 10 | M | R Hemi | 40 | 18 m | I | None | No | ham, gastr | 11 |
| 10 | 4 | F | R Hemi | 40 | ? | I | None | No | gastr, sol | 6.5 |
| 11 | 3 | M | R Hemi | ? | ? | I | None | No | ham, gastr, sol | 12 |
| 12 | 5 | F | R Hemi | 30 | 18 m | I | None | No | ham, gastr | 14 |
| 13 | 6 | M | L Hemi | 28 | ? | I | None | No | ham, gastr, sol | 15 |
| 14 | 4 | M | L Hemi | 40 | 12 m | II | None | No | add, ham, gastr, sol | 14 |
| 15 | 4 | F | Di | 39 | 15 m | I | Mild | No | ps, add, ham, gastr | 24 |
| 16 | 5 | M | Di | 35 | 16 m | II | None | No | ps, ham, gastr | 18 |
| 17 | 12 | F | Di | 33 | 24 m | II | Severe | No | ps, ham | 15 |
| 18 | 6 | M | Di | 35 | 11 m | II | None | No | ps, add, ham, gastr | 29 |
| 19 | 12 | M | Di | 26 | 24 m | II | Mild | No | ham, gastr | 18 |
| 20 | 8 | M | Di | 25 | 24 m | II | None | No | ps, add, ham, gastr | 15 |
| 21 | 3 | F | Di | 37 | 24 m | II | None | No | add, ham, gastr | 28 |
| 22 | 8 | M | Di | 41 | 27 m | I | None | No | ps, add, ham, gastr | 16 |
| 23 | 6 | M | Di | 40 | 20m | II | None | No | ham, gastr | 20 |
| 24 | 7 | M | Di | 40 | ? | II | None | No | add, ham, gastr | 17.5 |
| 25 | 9 | M | Di | 28 | ? | I | None | No | ps, add, ham, gastr | 21 |
| 26 | 6 | M | Di | 40 | 48 m | II | None | No | ham, gastr | 18 |
| 27 | 8 | M | Di | 40 | 30 m | II | None | No | add, ham | 16.5 |
| 28 | 10 | M | Di | ? | ? | I | None | No | ham, gastr | 16 |
4.2. Botulinum Toxin Injection
4.3. Locomotor Task and Recording

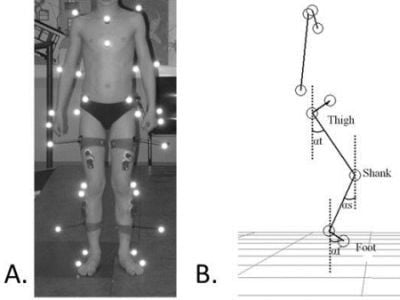
4.4. Kinematic Analysis

4.5. Ethical Aspects
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M. A report: The definition and classification of cerebral palsy. Dev. Med. Child Neurol. 2007, 109, 8–14. [Google Scholar]
- Himmelmann, K.; Hagberg, G.; Beckung, E.; Hagberg, B.; Uvebrant, P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995-1998. Acta Paediatr. 2005, 3, 287–294. [Google Scholar]
- Cobeljic, G.; Bumbasirevic, M.; Lesic, A.; Bajin, Z. The management of spastic equinus in cerebral palsy. Orthop. Trauma 2009, 23, 201–209. [Google Scholar] [CrossRef]
- Graham, H.K.; Aoki, K.R.; Autti-Rämö, I. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture 2000, 11, 67–79. [Google Scholar] [CrossRef]
- Sutherland, D.H.; Kaufman, K.R.; Wyatt, M.P.; Chambers, H.G. Injection of botulinum A toxin into the gastrocnemius muscle of patients with cerebral palsy: A 3-dimensional motion analysis study. Gait Posture 1996, 4, 269–227. [Google Scholar] [CrossRef]
- Mackey, A.; Walt, S.; Stott, N.S. Botulinum toxin type A in ambulant children with cerebral palsy. Physiotherapy 2003, 89, 219–232. [Google Scholar] [CrossRef]
- Molenaers, G.; Desloovere, K.; Fabry, G.; de Cock, P. The effects of quantitative gait assessment and botulinum Toxin A on musculoskeletal surgery in children with cerebral palsy. J. Bone Joint Surg. Am. 2006, 88, 161–170. [Google Scholar] [CrossRef]
- Bleyenheuft, C.; Cockx, S.; Caty, G.; Stoquart, G.; Lejeune, T.; Detrembleur, C. The effect of botulinum toxin injections on gait control in spastic stroke patients presenting with a stiff-knee gait. Gait Posture 2009, 30, 168–172. [Google Scholar] [CrossRef]
- Wong, V. Use of botulinum toxin injection in 17 children with spastic cerebral palsy. Eur. J. Paediatr. Neurol. 1998, 18, 124–131. [Google Scholar]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Brogren, E.; Katz-Salamon, M.; Forssberg, H. Periventricular leukomalacia and preterm birth have a detrimental effect on postural adjustments. Brain 1996, 122, 727–740. [Google Scholar]
- Hedberg, A.; Brogen-Carlberg, E.; Forssberg, H.; Hadders-Algra, M. Development of postural adjustments in sitting position during the first half year of life. Dev. Med. Child Neurol. 2005, 47, 312–320. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Development of postural control during the first 18 months of life. Neural Plast. 2005, 12, 99–108. [Google Scholar] [CrossRef]
- Brogren Carlberg, E.; Hadders-Algra, M. Postural Control in Sitting Children with Cerebral Palsy. In Postural Control: A key Issue in Developmental Disorders; Wiley-Blackwell: London, UK, 2008; pp. 74–96. [Google Scholar]
- Woollacott, M.H.; Burtner, P.; Jensen, J.; Jasiewicz, J.; Roncenvalles, N.; Sveistrup, H. Development of postural responses during standing in healthy children and children with spastic diplegia. Neurosci. Biobehav. Rev. 1998, 22, 583–589. [Google Scholar] [CrossRef]
- Bahramizadeh, M.; Mousavi, M.E.; Rassafiani, M.; Aminian, G.; Ebrahimi, I.; Karimlou, M.; Toole, G.O. The effect of floor reaction ankle foot orthosis on postural control in children with spastic cerebral palsy. Prosthet. Orthot. Int. 2012, 36, 71–76. [Google Scholar] [CrossRef]
- Hayek, S.; Hemo, Y.; Chamis, S.; Bat, R.; Segev, E.; Wientroub, S.; Yzhar, Z. The effect of community-prescribed ankle-foot orthoses on gait parameters in children with spastic cerebral palsy. J. Child Orthop. 2007, 1, 325–332. [Google Scholar] [CrossRef]
- Balaban, B.; Yasar, E.; Dal, U.; Yazicioglu, K.; Mohur, H.; Kalyon, T.A. The effect of hinged ankle-foot orthosis on gait and energy expenditure in spastic hemiplegic cerebral palsy. Disabil. Rehabil. 2007, 29, 139–144. [Google Scholar] [CrossRef]
- Molenaers, G.; Desloovere, K.; van Campenhout, A.; Pauwels, P.; Ortibus, E.; van de Walle, P. Effect of ankle foot orthoses on 3D trunk and pelvic motion during gait in children with CP. Gait Posture 2006, 24, S98–S289. [Google Scholar] [CrossRef]
- Degelaen, M.; de Borre, L.; Salvia, P.; Pelc, K.; Kerckhofs, E.; de Meirleir, L.; Cheron, G.; Dan, B. Effect of ankle-foot orthoses on trunk sway and lower limb intersegmental coordination in children with bilateral cerebral palsy. J. Ped. Rehabil. Med. 2012, 5, 171–179. [Google Scholar]
- Nahorniak, M.T.; Gorton, G.E., III; Gannotti, M.E.; Masso, P.D. Kinematic compensations as children reciprocally ascend and descend stairs with unilateral and bilateral solid AFOs. Gait Posture 1999, 9, 199–206. [Google Scholar] [CrossRef]
- Grasso, R.; Bianchi, L.; Lacquaniti, F. Motor patterns for human gait: Backward versus forward locomotion. J. Neurophysiol. 1998, 80, 1868–1885. [Google Scholar]
- Dan, B.; Bouillot, E.; Bengoetxea, A.; Cheron, G. Effect of intrathecal baclofen on gait control in human hereditary spastic paraparesis. Neurosci. Lett. 2000, 208, 175–178. [Google Scholar]
- Leurs, F.; Bengoetxea, A.; Cebolla, A.M.; de Saedeleer, C.; Dan, B.; Cheron, G. Planar covariation of elevation angles in prosthetic gait. Gait Posture 2012, 35, 647–652. [Google Scholar] [CrossRef]
- Cheron, G.; Bouillot, E.; Dan, B.; Bengoetxea, A.; Draye, J.P.; Lacquaniti, F. Development of a kinematic coordination pattern in toddler locomotion: Planar covariation. Exp. Brain Res. 2001, 137, 455–466. [Google Scholar] [CrossRef]
- Bianchi, L.; Angelini, D.; Orani, G.P.; Lacquaniti, F. Kinematic coordination in human gait: Relation to mechanical energy cost. J. Neurophysiol. 1998, 79, 2155–2170. [Google Scholar]
- Massin, M.; Allington, N. Role of exercise testing in the functional assessment of cerebral palsy children after Botulinum A toxin injection. J. Pediatr. Orthop. 1999, 19, 362–365. [Google Scholar]
- Balaban, B.; Fatih, T.; Kenan, T.A. Botulinum toxin A treatment in children with cerebral palsy: Its effects on walking and energy expenditure. Am. J. Phys. Med. Rehabil. 2012, 91, 53–64. [Google Scholar] [CrossRef]
- Fowler, E.G.; Goldberg, E.J. The effect of lower extremity selective voluntary motor control on interjoint coordination during gait in children with spastic diplegic cerebral palsy. Gait Posture 2009, 29, 102–107. [Google Scholar] [CrossRef]
- Østensjø, S.; Brogren Carlberg, E.; Vøllestad, N.K. Motor impairments in young children with cerebral palsy: Relationship to gross motor function and everyday activities. Dev. Med. Child. Neur. 2004, 46, 580–589. [Google Scholar]
- Degelaen, M.; Leurs, F.; de Borre, L.; Kerckhofs, E.; de Meirleir, L.; Cheron, G.; Dan, B. Effect of ankle-foot orthoses in gait in typically developing children: Development trend in segmental coordination. J. Ped. Rehabil. Med. 2010, 3, 163–170. [Google Scholar]
- Molenaers, G.; van Campenhout, A.; Fagard, K.; de Cat, J.; Desloovere, K. The use of botulinum toxin A in children with cerebral palsy with a focus on the lower limb. J. Child Orthop. 2010, 4, 183–195. [Google Scholar]
- Borghese, N.A.; Bianchi, L.; Lacquaniti, F. Kinematic determinants of human locomotion. J. Physiol. 1996, 494, 863–879. [Google Scholar]
- Cheron, G.; Bengoetxea, A.; Bouillot, E.; Lacquaniti, F.; Dan, B. Early emergence of temporal co-ordination of lower limb segments elevation angles in human locomotion. Neurosci. Lett. 2001, 308, 123–127. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Dominici, N.; Cappellini, G.; Dan, B.; Cheron, G.; Lacquaniti, F. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. J. Exp. Biol. 2004, 207, 3797–3810. [Google Scholar] [CrossRef]
- Lacquaniti, F.; Grasso, R.; Zago, M. Motor patterns in walking. News Physiol. Sci. 1999, 14, 168–174. [Google Scholar]
- Ivanenko, Y.P.; D’Avella, A.; Poppele, R.E.; Lacquaniti, F. On the origin of planar covariation of elevation angles during human locomotion. J. Neurophysiol. 2008, 99, 1890–1898. [Google Scholar] [CrossRef]
- Barliya, A.; Omlor, L.; Giese, M.A.; Flash, T. An analytical formulation of the law of intersegmental coordination during human locomotion. Exp. Brain Res. 2009, 193, 371–385. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Cappellini, G.; Dominici, N.; Poppele, R.E.; Lacquaniti, F. Modular control of limb movements during human locomotion. J. Neurosci. 2007, 27, 11149–11161. [Google Scholar]
- Winstein, C.J.; Garfinkel, A. Qualitative dynamics of disordered human locomotion: A preliminary investigation. J. Mot. Behav. 1989, 4, 373–391. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Degelaen, M.; De Borre, L.; Kerckhofs, E.; De Meirleir, L.; Buyl, R.; Cheron, G.; Dan, B. Influence of Botulinum Toxin Therapy on Postural Control and Lower Limb Intersegmental Coordination in Children with Spastic Cerebral Palsy. Toxins 2013, 5, 93-105. https://doi.org/10.3390/toxins5010093
Degelaen M, De Borre L, Kerckhofs E, De Meirleir L, Buyl R, Cheron G, Dan B. Influence of Botulinum Toxin Therapy on Postural Control and Lower Limb Intersegmental Coordination in Children with Spastic Cerebral Palsy. Toxins. 2013; 5(1):93-105. https://doi.org/10.3390/toxins5010093
Chicago/Turabian StyleDegelaen, Marc, Ludo De Borre, Eric Kerckhofs, Linda De Meirleir, Ronald Buyl, Guy Cheron, and Bernard Dan. 2013. "Influence of Botulinum Toxin Therapy on Postural Control and Lower Limb Intersegmental Coordination in Children with Spastic Cerebral Palsy" Toxins 5, no. 1: 93-105. https://doi.org/10.3390/toxins5010093
APA StyleDegelaen, M., De Borre, L., Kerckhofs, E., De Meirleir, L., Buyl, R., Cheron, G., & Dan, B. (2013). Influence of Botulinum Toxin Therapy on Postural Control and Lower Limb Intersegmental Coordination in Children with Spastic Cerebral Palsy. Toxins, 5(1), 93-105. https://doi.org/10.3390/toxins5010093