Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli
Abstract
:1. Introduction
2. Diarrhea and ETEC
3. Enterotoxins
Relation between LT and CT

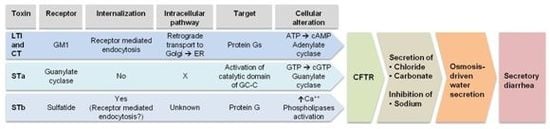
4. Mechanism of Action
4.1. LT
4.2. STa
4.3. STb

5. Antibacterial Activities toward ETEC

Examples of Antibacterial Activities Related to Plant Products
| Plant | Scientific name | Specific compound | Target | Mechanism | Reference |
|---|---|---|---|---|---|
| Apple | Malus spp | Applephenon | V. cholerae | Inhibits CT ADP-ribosylation activity | [75] |
| Bean | Vicia faba | ? | ETEC | Inhibits LT binding to GM1 | [115,116] |
| Berberine* | Berberis aristata | Alkaloid | ETEC and V. cholerae | Effect on tight jonctions, NHE3 and AQP4 Inhibits secretory response of STa | [128] |
| Black tea* | Camellia sinensis | Catechins (EGCG) | ETEC | ? | [69] |
| Cocoa | Theobroma cacao | Flavonoids | ETEC | Inhibits CFTR | [142] |
| Daio (kampo formulation) | Rhei rhizoma | Polygallate (rhubarb galloyl tannin) | V. cholerae | Inhibits CT ADP-ribosylation actvity | [76] |
| Elephant garlic | Allium ampeloprasum | Diallyl sulfides | V. cholerae | Growth inhibition | [39] |
| Fenugreek | Trigonella foenum-graecum | Galactomannans | ETEC and V. cholerae | Inhibits LT and CT binding to GM1 | [148] |
| Flor de manita | Chiranthodendron pentadactylon | (−) Epicatechin | ETEC and V. cholerae | Interacts with CTA subunit | [144] |
| Flowering quince | Chaenomeles speciosa | Oleanic acid, ursolic acid, betulinic acid | ETEC | Inhibits LTB binding to GM1 | [147] |
| Gall of R. sinensis* | Galla sinensis | Gallic acid (methyl gallate) | ETEC | Inhibits binding of LTB to GM1 | [54] |
| Garlic | Allium sativum | Diallyl sulfides | V. cholerae | Growth inhibition | [39] |
| Ginger | Zingiber officinale | Zingerone and zingerol | ETEC | Inhibits LTB binding to GM1 | [110] |
| Green tea* | Camellia sinensis | (−)-(Epigallocatechin-3-gallate), gallotanins | ETEC and V. cholerae | Inhibits calcium chloride channels | [73] |
| Guazyma | Guazyma ulmifolia | Procyanidins | V. cholerae | Interacts with CTA subunit | [146] |
| Hop | Humulus lupulus | Procyanidins | V. cholerae | Inhibits CT ADP-ribosylation activity | [74] |
| Kampo formulations | Hange-Shashin-to (TJ-14), Keishi-Ka-Shakuyaku-to (TJ-60) | ? | ETEC | TJ-14 and TJ-60 suppress colon contractions TJ-60 blocks GCC activity (STa) | [77] |
| Liquorice | Glycyrrhiza uralensis | Glycyrrhizin (oleane-type triterpenoids) | ETEC | Inhibits LTB-GM1 interaction | [114] |
| Neem | Azadirachta indica | ? | V. cholerae | Growth inhibition and antisecretory activity | [41] |
| Palmarosa | Cymbopogon martinii | Geraniol | ETEC | Growth inhibition | [32] |
| Pea | Pisum sativum | ? | ETEC | Inhibits LT binding to GM1 | [116,117] |
| Pepper* | Piper longum | Piperine | ETEC and V. cholerae | Antibacterial | [139] |
| Red chili* | Capsicum annuum | Capsaicin | V. cholerae | Inhibits CT production | [6] |
| Red seaweeds | Gigartina sp. | λ carragenin | ETEC | Mimicks STb receptor | [149] |
| Sangre de drago | Croton lechleri | Crofelemer | ETEC | Inhibits CFTR and calcium-activated chloride channels | [89] |
| Sumac* | Rhus sinensis | Gallotannins | ETEC | Antibacterial | [52] |
| Tempe | Glycine max | Arabinose-containing molecule | ETEC | Inhibits adhesion of F4 | [47] |
| Wood creosote | Fagus crenata | Seirogan | ETEC | Antisecretory and antimotility (STa and LT) | [105] |
6. Tempe
7. Sumac
8. Tea and Tea Extracts
9. Plant Polyphenols, Rhubarb Galloyl-Tannin and Applephenon
10. Kampo

11. Sangre de Drago
12. Wood Creosote
13. Red Chili
14. Ginger
15. Licorice
16. Pea and Fava Bean Hulls
17. Berberine
18. Piperine
19. Cocoa
20. “Flor de Manita” or Macpaxochitl
21. Flowering Quince or Chanomeles
22. Fenugreek
23. Algae
24. Conclusions
Acknowledgments
Conflicts of Interest
References
- Palombo, E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: Modes of action and effects on intestinal function. Phytother. Res. 2006, 20, 717–724. [Google Scholar] [CrossRef]
- Solecki, R.S. Shanidar IV, a neanderthal flower burial in northern Iraq cave. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Kim, H.S. Do not put too much value on conventional medicines. J. Ethnopharmacol. 2005, 100, 37–39. [Google Scholar] [CrossRef]
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Groombridge, B.J.; Jenkins, M.D. World Atlas of Biodiversity: Earth’s Living Resources in the 21st Century; University of California Press: Berkeley, CA, USA, 2002. [Google Scholar]
- Chatterjee, S.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Sugimoto, N.; Haldar, S.; Awasthi, S.P.; Hinenoya, A.; Aoki, S.; Yamasaki, S. Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Microbiol. Lett. 2010, 306, 54–60. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Namkung, W.; Verkman, A.S. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol. Pharmacol. 2010, 77, 69–78. [Google Scholar] [CrossRef]
- World Health Organisation, State of the Art of New Vaccines: Research in Development. Diarrhoeal Diseases; WHO: Geneva, Switzerland, 2005.
- Kosek, M.; Bern, C.; Guerrant, R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar]
- Dubreuil, J.D. The whole Shebang: The gastrointestinal tract, Escherichia coli enterotoxins and secretion. Curr. Issues Mol. Biol. 2012, 14, 71–82. [Google Scholar]
- Osek, J. Prevalence of virulence factors of Escherichia coli strains isolated from diarrheic and healthy piglets after weaning. Vet. Microbiol. 1999, 68, 209–217. [Google Scholar] [CrossRef]
- Honda, T.; Takeda, Y.; Miwatani, T. Isolation of special antibodies which react only with homologous enterotoxins from Vibrio cholerae and Enterotoxigenic Escherichia coli. Infect. Immun. 1981, 34, 333–336. [Google Scholar]
- Mainil, J. Escherichia coli virulence factors. Vet. Immunol. Immunopathol. 2012, 152, 2–12. [Google Scholar] [CrossRef]
- Nair, G.B.; Takeda, Y. The heat-stable enterotoxins. Microb. Pathog. 1998, 24, 123–131. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Escherichia coli STb toxin and colibacillosis: Knowing is half the battle. FEMS Microbiol. Lett. 2008, 278, 137–145. [Google Scholar] [CrossRef]
- Lortie, L.A.; Dubreuil, J.D.; Harel, J. Characterization of Escherichia coli strains producing heat-stable enterotoxin b (STb) isolated from humans with diarrhea. J. Clin. Microbiol. 1991, 29, 656–659. [Google Scholar]
- Okamoto, K.; Fujii, Y.; Akashi, N.; Hitotsubashi, S.; Kurazono, H.; Karasawa, T.; Takeda, Y. Identification and characterization of heat-stable enterotoxin II-producing Escherichia coli from patients with diarrhea. Microbiol. Immunol. 1993, 37, 411–414. [Google Scholar]
- Dallas, W.S.; Falkow, S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature 1980, 288, 499–501. [Google Scholar] [CrossRef]
- Lencer, W.I.; Constable, C.; Moe, S.; Rufo, P.A.; Wolf, A.; Jobling, M.G.; Ruston, S.P.; Madara, J.L.; Holmes, R.K.; Hirst, T.R. Proteolytic activation of cholera toxin and Escherichia coli labile toxin by entry into host epithelial cells. Signal transduction by a protease-resistant toxin variant. J. Biol. Chem. 1997, 272, 15562–15568. [Google Scholar] [CrossRef]
- Spangler, B.D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 1992, 56, 622–647. [Google Scholar]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar]
- Rappuoli, R.; Pizza, M.; Douce, G.; Dougan, G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 1999, 20, 493–500. [Google Scholar] [CrossRef]
- Vaandrager, A.B. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol. Cell. Biochem. 2002, 230, 73–83. [Google Scholar] [CrossRef]
- Hitotsubashi, S.; Fujii, Y.; Yamanaka, H.; Okamoto, K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect. Immun. 1992, 60, 4468–4474. [Google Scholar]
- Rousset, E.; Harel, J.; Dubreuil, J.D. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 1998, 66, 5650–5658. [Google Scholar]
- Labrie, V.; Harel, J.; Dubreuil, J.D. Escherichia coli heat-stable enterotoxin b (STb) in vivo internalization within rat intestinal epithelial cells. Vet. Res. 2002, 33, 223–228. [Google Scholar] [CrossRef]
- Dreyfus, L.A.; Harville, B.; Howard, D.E.; Shaban, R.; Beatty, D.M.; Morris, S.J. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB). Proc. Natl. Acad. Sci. USA 1993, 90, 3202–3206. [Google Scholar] [CrossRef]
- Hitotsubashi, S.; Akagi, M.; Saitou, A.; Yamanaka, H.; Fujii, Y.; Okamoto, K. Action of Escherichia coli heat-stable enterotoxin II on isolated sections of mouse ileum. FEMS Microbiol. Lett. 1992, 69, 249–252. [Google Scholar]
- Harville, B.A.; Dreyfus, L.A. Involvement of 5-hydroxytryptamine and prostaglandin E2 in the intestinal secretory action of Escherichia coli heat-stable enterotoxin B. Infect. Immun. 1995, 63, 745–750. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar]
- Duarte, M.C.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef]
- Taylor, P.W. Alternative natural sources for a new generation of antibacterial agents. Int. J. Antimicrob. Agents 2013.
- Dog, T.L. A reason to season: The therapeutic benefits of spices and culinary herbs. Explore NY 2006, 2, 446–449. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Yamasaki, S.; Asakura, M.; Neogi, S.B.; Hinenoya, A.; Iwaoka, E.; Aoki, S. Inhibition of virulence potential of Vibrio cholerae by natural compounds. Indian J. Med. Res. 2011, 133, 232–239. [Google Scholar]
- Ahn, Y.J.; Lee, C.O.; Kweon, J.H.; Ahn, J.W.; Park, J.H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. J. Appl. Microbiol. 1998, 84, 439–443. [Google Scholar]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial activity of elephant garlic oil against Vibrio cholerae in vitro and in a food model. Biosci. Biotechnol. Biochem. 2009, 73, 1623–1627. [Google Scholar] [CrossRef]
- Hersch-Martinez, P.; Leanos-Miranda, B.E.; Solorzano-Santos, F. Antibacterial effects of commercial essential oils over locally prevalent pathogenic strains in Mexico. Fitoterapia 2005, 76, 453–457. [Google Scholar] [CrossRef]
- Thakurta, P.; Bhowmik, P.; Mukherjee, S.; Hajra, T.K.; Patra, A.; Bag, P.K. Antibacterial, antisecretory and antihemorrhagic activity of Azadirachta indica used to treat cholera and diarrhea in India. J. Ethnopharmacol. 2007, 111, 607–612. [Google Scholar] [CrossRef]
- Roubos-van den Hil, P.J.; Nout, M.J.; van der Meulen, J.; Gruppen, H. Bioactivity of tempe by inhibiting adhesion of ETEC to intestinal cells, as influenced by fermentation substrates and starter pure cultures. Food Microbiol. 2010, 27, 638–644. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, M.J.; Rombouts, F.M.; Nabuurs, M.J.; van der Meulen, J. Inhibition of adhesion of enterotoxigenic Escherichia coli K88 by soya bean tempe. Lett. Appl. Microbiol. 2002, 35, 311–315. [Google Scholar] [CrossRef]
- Kiers, J.L.; Meijer, J.C.; Nout, M.J.; Rombouts, F.M.; Nabuurs, M.J.; van der Meulen, J. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J. Appl. Microbiol. 2003, 95, 545–552. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, M.J.; Rombouts, F.M.; van Andel, E.E.; Nabuurs, M.J.; van der Meulen, J. Effect of processed and fermented soyabeans on net absorption in enterotoxigenic Escherichia coli-infected piglet small intestine. Br. J. Nutr. 2006, 95, 1193–1198. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, M.J.; Rombouts, F.M.; Nabuurs, M.J.; van der Meulen, J. A high molecular weight soluble fraction of tempeh protects against fluid losses in Escherichia coli-infected piglet small intestine. Br. J. Nutr. 2007, 98, 320–325. [Google Scholar] [CrossRef]
- Roubos-van den Hil, P.J.; Nout, M.J.; Beumer, R.R.; van der Meulen, J.; Zwietering, M.H. Fermented soya bean (tempe) extracts reduce adhesion of enterotoxigenic Escherichia coli to intestinal epithelial cells. J. Appl. Microbiol. 2009, 106, 1013–1021. [Google Scholar] [CrossRef]
- Roubos-van den Hil, P.J.; Schols, H.A.; Nout, M.J.; Zwietering, M.H.; Gruppen, H. First characterization of bioactive components in soybean tempe that protect human and animal intestinal cells against enterotoxigenic Escherichia coli (ETEC) infection. J. Agric. Food Chem. 2010, 58, 7649–7656. [Google Scholar] [CrossRef]
- Mo, H.; Zhu, Y.; Nout, M.J. In vitro digestion enhances anti-adhesion effect of tempe and tofu against Escherichia coli. Lett. Appl. Microbiol. 2011, 54, 166–168. [Google Scholar]
- Miller, A.J.; Young, D.A.; Miller, A.J.; Wen, J. Phylogeny and biogeography of Rhus (Anacardiaceae) based on ITS sequence data. Int. J. Plant Sci. 2001, 162, 1401–1407. [Google Scholar] [CrossRef]
- Rayne, S.; Mazza, G. Biological activities of extracts from sumac (Rhus spp.): A review. Plant Foods Hum. Nutr. 2007, 62, 165–175. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis—Folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, T.Y.; Chang, Y.S.; Wu, S.L.; Hsiang, C.Y. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. J. Ethnopharmacol. 2006, 103, 385–391. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Shin, D.W.; Kwon, D.Y. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef]
- Tangpu, V.; Yadav, A.K. Antidiarrhoeal activity of Rhus javanica ripen fruit extract in albino mice. Fitoterapia 2004, 75, 39–44. [Google Scholar] [CrossRef]
- Bose, S.K.; Dewanjee, S.; Gupta, A.S.; Samanta, K.C.; Kundu, M.; Mandal, S.C. In vivo evaluation of antidiarrhoeal activity of Rhus semialata fruit extracts in rats Afr. J. Trad. CAM 2008, 5, 97–102. [Google Scholar]
- Bruins, M.J.; Cermak, R.; Kiers, J.L.; van der Meulen, J.; van Amelsvoort, J.M.; van Klinken, B.J. In vivo and in vitro effects of tea extracts on enterotoxigenic Escherichia coli-induced intestinal fluid loss in animal models. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 459–469. [Google Scholar] [CrossRef]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ikigai, H.; Suzuki, T.; Suzuki, Y.; Hara, Y.; Shimamura, T. The protective activity of tea catechins against experimental infection by Vibrio cholerae O1. Microbiol. Immunol. 1992, 36, 999–1001. [Google Scholar]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E.; Kozukue, N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 2006, 69, 354–361. [Google Scholar]
- Caturla, N.; Vera-Samper, E.; Villalain, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- Nakayama, T.; Hashimoto, T.; Kajiya, K.; Kumazawa, S. Affinity of polyphenols for lipid bilayers. Biofactors 2000, 13, 147–151. [Google Scholar] [CrossRef]
- Abboud, P.A.; Hake, P.W.; Burroughs, T.J.; Odoms, K.; O’Connor, M.; Mangeshkar, P.; Wong, H.R.; Zingarelli, B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur. J. Pharmacol. 2008, 579, 411–417. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T. Selection of Escherichia coli heat-labile toxin (LT) inhibitors using both the GM1-ELISA and the cAMP vero cell assay. Foodborne Pathog. Dis. 2013, 10, 603–607. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T.A. E. coli heat labile toxin (LT) inactivation by specific polyphenols is aggregation dependent. Vet. Microbiol. 2013, 163, 319–324. [Google Scholar] [CrossRef]
- Besra, S.E.; Gomes, A.; Ganguly, D.K.; Vedasiromoni, J.R. Antidiarrhoeal activity of hot water extract of black tea (Camellia sinensis). Phytother. Res. 2003, 17, 380–384. [Google Scholar] [CrossRef]
- Bruins, M.J.; Vente-Spreeuwenberg, M.A.; Smits, C.H.; Frenken, L.G. Black tea reduces diarrhoea prevalence but decreases growth performance in enterotoxigenic Escherichia coli-infected post-weaning piglets. J. Anim. Physiol. Anim. Nutr. Berl 2011, 95, 388–398. [Google Scholar] [CrossRef]
- Ishihara, N.; Akachi, S. Green tea extract as a remedy for diarrhea in far-raised calves. In Chemistry and Applications of Green Tea; Yamamoto, T., Jejuna, L.R., Chu, D.C., Kims, M., Eds.; CRC Press LLC: Boca Raton, FL, USA, 1997; pp. 137–144. [Google Scholar]
- Ishihara, N.; Chu, D.C.; Akachi, S.; Juneja, L.R. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest. Prod. Sci. 2001, 87, 382–386. [Google Scholar]
- Ngure, K.M.; Wanyoko, j.K.; Mahungu, S.M.; Shitandi, A.A. Catechins depeltion patterns in relation to theaflavin and thearubigins formation. Food Chem. 2009, 115, 8–14. [Google Scholar] [CrossRef]
- Namkung, W.; Thiagarajah, J.R.; Phuan, P.W.; Verkman, A.S. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010, 24, 4178–4186. [Google Scholar] [CrossRef]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef]
- Saito, T.; Miyake, M.; Toba, M.; Okamatsu, H.; Shimizu, S.; Noda, M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol. Immunol. 2002, 46, 249–255. [Google Scholar]
- Oi, H.; Matsuura, D.; Miyake, M.; Ueno, M.; Takai, I.; Yamamoto, T.; Kubo, M.; Moss, J.; Noda, M. Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 3042–3046. [Google Scholar]
- Kito, Y.; Teramoto, N. Effects of Hange-shashin-to (TJ-14) and Keishi-ka-shakuyaku-to (TJ-60) on contractile activity of circular smooth muscle of the rat distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1059–G1066. [Google Scholar] [CrossRef]
- Jones, K. Review of sangre de drago (Croton lechleri)—A South American tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: traditional uses to clinical research. J. Altern. Complement. Med. 2003, 9, 877–896. [Google Scholar] [CrossRef]
- Peres, M.T.; Delle Monache, F.; Cruz, A.B.; Pizzolatti, M.G.; Yunes, R.A. Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 1997, 56, 223–226. [Google Scholar] [CrossRef]
- Peres, M.T.L.P.; Delle Monache, F.; Pizzolatti, M.G.; Santos, A.R.S.; Beirith, A.; Calixto, J.B.; Yunes, R.A. Analgesic compounds of Croton urucurana Baillon Baillon. Pharmacochemical criteria used in their isolation. Phytother. Res. 1998, 12, 209–222. [Google Scholar] [CrossRef]
- Gurgel, L.A.; Silva, R.M.; Santos, F.A.; Martins, D.T.; Mattos, P.O.; Rao, V.S. Studies on the antidiarrhoeal effect of dragon’s blood from Croton urucurana. Phytother. Res. 2001, 15, 319–322. [Google Scholar] [CrossRef]
- Rao, V.S.; Gurgel, L.A.; Lima-Junior, R.C.; Martins, D.T.; Cechinel-Filho, V.; Santos, F.A. Dragon’s blood from Croton urucurana (Baill.) attenuates visceral nociception in mice. J. Ethnopharmacol. 2007, 113, 357–360. [Google Scholar] [CrossRef]
- Miller, M.J.; MacNaughton, W.K.; Zhang, X.J.; Thompson, J.H.; Charbonnet, R.M.; Bobrowski, P.; Lao, J.; Trentacosti, A.M.; Sandoval, M. Treatment of gastric ulcers and diarrhea with the Amazonian herbal medicine sangre de grado. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G192–G200. [Google Scholar]
- Ubillas, R.; Jolad, S.D.; Bruening, R.C.; Kernan, M.R.; King, S.R.; Sesin, D.F.; Barrett, M.; Stoddart, C.A.; Flaster, T.; Kuo, J.; et al. SP-303, an antiviral oligomeric proanthocyanidin from the latex of Croton lechleri (Sangre de Drago). Phytomedicine 1994, 1, 77–106. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Davenport, S.E.; Steagall, R.J.; Vimal, V.; Carlson, T.; Rozhon, E.J. A novel plant-derived inhibitor of cAMP-mediated fluid and chloride secretion. Am. J. Physiol. 1999, 276, G58–G63. [Google Scholar]
- DiCesare, D.; DuPont, H.L.; Mathewson, J.J.; Ashley, D.; Martinez-Sandoval, F.; Pennington, J.E.; Porter, S.B. A double blind, randomized, placebo-controlled study of SP-303 (Provir) in the symptomatic treatment of acute diarrhea among travelers to Jamaica and Mexico. Am. J. Gastroenterol. 2002, 97, 2585–2588. [Google Scholar] [CrossRef]
- Holodniy, M.; Koch, J.; Mistal, M.; Schmidt, J.M.; Khandwala, A.; Pennington, J.E.; Porter, S.B. A double blind, randomized, placebo-controlled phase II study to assess the safety and efficacy of orally administered SP-303 for the symptomatic treatment of diarrhea in patients with AIDS. Am. J. Gastroenterol. 1999, 94, 3267–3273. [Google Scholar] [CrossRef]
- Fischer, H.; Machen, T.E.; Widdicombe, J.H.; Carlson, T.J.; King, S.R.; Chow, J.W.; Illek, B. A novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cells. J. Ethnopharmacol. 2004, 93, 351–357. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Namkung, W.; Verkman, A.S. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol. Pharmacol. 2009, 77, 69–78. [Google Scholar] [CrossRef]
- Cottreau, J.; Tucker, A.; Crutchley, R.; Garey, K.W. Crofelemer for the treatment of secretory diarrhea. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 17–23. [Google Scholar] [CrossRef]
- Bardhan, P.K.; Sharma, A.; Bolmall, C.; et al. Safety and efficacy of a novel anti-secretory anti-diarrheal agent crofelemer (NP-303),in the treatment of adult acute infectious diarrhea and cholera, with or without the use of antibiotics. In Proceedings of US-Japan CMSP: 13th International Conference on emerging infectious diseases (EID) in the pacific rim-Focused on enteric diseases, Kolkata, India, 6–9 April 2009; Volume 40, pp. 1065–1073.
- Greenwood-Van Meerveld, B.; Tyler, K.; Kuge, T.; Ogata, N. Anti-diarrhoeal effects of seirogan in the rat small intestine and colon examined in vitro. Aliment. Pharmacol. Ther. 1999, 13, 97–102. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Tyler, K.R.; Venkova, K.; Kuge, T. Comparison of the antidiarrheal effects of wood creosote and loperamide in the rat jejunum and colon in vitro. Biol. Pharm. Bull. 2000, 23, 952–956. [Google Scholar] [CrossRef]
- Ataka, K.; Ogata, N.; Kuge, T.; Shibata, T. Suppression of enterotoxin-induced intestinal fluid secretion by wood creosote. Res. Commun. Mol. Pathol. Pharmacol. 1996, 93, 219–224. [Google Scholar]
- Kuge, T.; Venkova, K.; Greenwood-Van Meerveld, B. In vitro effects of wood creosote on enterotoxin-induced secretion measured electrophysiologically in the rat jejunum and colon. Biol. Pharm. Bull. 2001, 24, 623–627. [Google Scholar] [CrossRef]
- Ogata, N.; Shibata, T. Antidiarrheal activity of wood creosote: inhibition of muscle contraction and enterotoxin-induced fluid secretion in rabbit small intestine. Pharmacology 2001, 62, 181–187. [Google Scholar] [CrossRef]
- Ataka, K.; Kuge, T.; Venkova, K.; Greenwood-Van Meerveld, B. Seirogan (wood creosote) inhibits stress-induced ion secretion in rat intestinal epithelium. Dig. Dis. Sci. 2003, 48, 1303–1309. [Google Scholar] [CrossRef]
- Kuge, T.; Greenwood-Van Meerveld, B.; Sokabe, M. Stress-induced breakdown of intestinal barrier function in the rat: Reversal by wood creosote. Life Sci. 2006, 79, 913–918. [Google Scholar] [CrossRef]
- Ogata, N.; Toyoda, M.; Shibata, T. Suppression of intestinal smooth muscle contraction by phenolic compounds. Res. Commun. Chem. Pathol. Pharmacol. 1992, 77, 359–366. [Google Scholar]
- Ogata, N.; Ataka, K.; Morino, H.; Shibata, T. Effect of wood creosote and loperamide on propulsive motility of mouse colon and small intestine. Pharmacology 1999, 59, 212–220. [Google Scholar] [CrossRef]
- Ataka, K.; Kuge, T.; Fujino, K.; Takahashi, T.; Fujimiya, M. Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton. Neurosci. 2007, 133, 136–145. [Google Scholar] [CrossRef]
- Kuge, T.; Venkova, K.; Greenwood-Van Meerveld, B. Effects of seirogan (wood creosote) on propulsive colonic motility and stool characteristics in ambulatory mini-pigs. Dig. Dis. Sci. 2002, 47, 2651–2656. [Google Scholar] [CrossRef]
- Kuge, T.; Shibata, T.; Willett, M.S. Wood creosote, the principal active ingredient of seirogan, an herbal antidiarrheal medicine: A single-dose, dose-escalation safety and pharmacokinetic study. Pharmacotherapy 2003, 23, 1391–1400. [Google Scholar] [CrossRef]
- Kuge, T.; Shibata, T.; Willett, M.S. Multiple-dose escalation, safety, and tolerability study of wood creosote, the principal active ingredient of seirogan, an herbal antidiarrheal medication, in healthy subjects. J. Clin. Pharmacol. 2003, 43, 284–290. [Google Scholar] [CrossRef]
- Ataka, K.; Ito, M.; Shibata, T. New views on antidiarrheal effect of wood creosote: Is wood creosote really a gastrointestinal antiseptic? Yakugaku Zasshi 2005, 125, 937–950. [Google Scholar] [CrossRef]
- DiRita, V.J.; Parsot, C.; Jander, G.; Mekalanos, J.J. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1991, 88, 5403–5407. [Google Scholar]
- Hase, C.C.; Mekalanos, J.J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1998, 95, 730–734. [Google Scholar] [CrossRef]
- Nye, M.B.; Pfau, J.D.; Skorupski, K.; Taylor, R.K. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 2000, 182, 4295–4303. [Google Scholar] [CrossRef]
- Borrelli, F.; Capasso, R.; Pinto, A.; Izzo, A.A. Inhibitory effect of ginger (Zingiber officinale) on rat ileal motility in vitro. Life Sci. 2004, 74, 2889–2896. [Google Scholar] [CrossRef]
- Chen, J.C.; Huang, L.J.; Wu, S.L.; Kuo, S.C.; Ho, T.Y.; Hsiang, C.Y. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J. Agric. Food Chem. 2007, 55, 8390–8397. [Google Scholar] [CrossRef]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shima, T.; Takewaki, T.; Shimizu, Y. Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J. Nat. Med. 2011, 65, 89–94. [Google Scholar] [CrossRef]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shimizu, Y. Intraluminal administration of zingerol, a non-pungent analogue of zingerone, inhibits colonic motility in rats. Biomed. Res. 2011, 32, 181–185. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, T.Y.; Chang, Y.S.; Wu, S.L.; Li, C.C.; Hsiang, C.Y. Identification of Escherichia coli enterotoxin inhibitors from traditional medicinal herbs by in silico, in vitro, and in vivo analyses. J. Ethnopharmacol. 2009, 121, 372–378. [Google Scholar] [CrossRef]
- Becker, P.M.; van der Meulen, J.; Jansman, A.J.; van Wikselaar, P.G. In vitro inhibition of ETEC K88 adhesion by pea hulls and of LT enterotoxin binding by faba bean hulls. J. Anim. Physiol. Anim. Nutr. Berl 2012, 96, 1121–1126. [Google Scholar] [CrossRef]
- Van der Meulen, J.; Jansman, A.J.M. Effect of pea and faba beans fractions on net fluid absorption in ETEC-infected small intestinal segments of weaned piglets. Livest. Sci. 2010, 133, 207–209. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T. The effects of plant polyphenols on enterotoxigenic Escherichia coli adhesion and toxin binding. Livest. Sci. 2010, 133, 101–103. [Google Scholar] [CrossRef]
- Sack, R.B.; Froehlich, J.L. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun. 1982, 35, 471–475. [Google Scholar]
- Sun, D.; Courtney, H.S.; Beachey, E.H. Berberine sulfate blocks adherence of Streptococcus pyogenes to epithelial cells, fibronectin, and hexadecane. Antimicrob. Agents Chemother. 1988, 32, 1370–1374. [Google Scholar] [CrossRef]
- Joshi, P.V.; Shirkhedkar, A.A.; Prakash, K.; Maheshwari, V.L. Antidiarrheal activity, chemical and toxicity profile of Berberis aristata. Pharm. Biol. 2011, 49, 94–100. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Patra, P.H.; Mahanti, A.; Mondal, D.K.; Dandapat, P.; Samanta, I.; Lodh, C.; Bera, A.K.; Bhattacharyya, D.; Sarkar, M.; et al. Potential antibacterial activity of berberine against multi drug resistant enterovirulent Escherichia coli isolated from yaks (Poephagus grunniens) with haemorrhagic diarrhoea. Asian Pac. J. Trop. Med. 2013, 6, 315–319. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Biswas, T.K.; Sasmal, D.; Samanta, I.; Gohosh, M.K. Evaluation of methanolic extract of Allium sativum and Saussurea costus in yaks with infectious keraconjunctivitis. Indian J. Anim. Sci. 2010, 80, 199–202. [Google Scholar]
- Islam, M.M.; Chowdhury, S.R.; Kumar, G.S. Spectroscopic and calorimetric studies on the binding of alkaloids berberine, palmatine and coralyne to double stranded RNA polynucleotides. J. Phys. Chem. B 2009, 113, 1210–1224. [Google Scholar]
- Gu, L.; Li, N.; Li, Q.; Zhang, Q.; Wang, C.; Zhu, W.; Li, J. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia 2009, 80, 241–248. [Google Scholar] [CrossRef]
- Pan, G.Y.; Wang, G.J.; Liu, X.D.; Fawcett, J.P.; Xie, Y.Y. The involvement of P-glycoprotein in berberine absorption. Pharmacol. Toxicol. 2002, 91, 193–197. [Google Scholar] [CrossRef]
- Taylor, C.T.; Baird, A.W. Berberine inhibition of electrogenic ion transport in rat colon. Br. J. Pharmacol. 1995, 116, 2667–2672. [Google Scholar] [CrossRef]
- Amasheh, M.; Fromm, A.; Krug, S.M.; Amasheh, S.; Andres, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J. Cell. Sci. 2010, 123, 4145–4155. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Sha, S.; Liang, S.; Zhao, L.; Liu, L.; Chai, N.; Wang, H.; Wu, K. Berberine increases the expression of NHE3 and AQP4 in sennosideA-induced diarrhoea model. Fitoterapia 2012, 83, 1014–1022. [Google Scholar] [CrossRef]
- Taylor, C.T.; Winter, D.C.; Skelly, M.M.; O'Donoghue, D.P.; O'Sullivan, G.C.; Harvey, B.J.; Baird, A.W. Berberine inhibits ion transport in human colonic epithelia. Eur. J. Pharmacol. 1999, 368, 111–118. [Google Scholar] [CrossRef]
- Zhou, H.; Mineshita, S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J. Pharmacol. Exp. Ther. 2000, 294, 822–829. [Google Scholar]
- Cernakova, M.; Kostalova, D. Antimicrobial activity of berberine—A constituent of Mahonia aquifolium. Folia Microbiol. Praha 2002, 47, 375–378. [Google Scholar] [CrossRef]
- Shin, D.H.; Yu, H.; Hsu, W.H. A paradoxical stimulatory effect of berberine on guinea-pig ileum contractility: Possible involvement of acetylcholine release from the postganglionic parasympathetic nerve and cholinesterase inhibition. Life Sci. 1993, 53, 1495–1500. [Google Scholar] [CrossRef]
- Birdsall, T.C.; Kelly, G.S. Berberine: Therapeutic potentail of an alkaloid found in several medicinal plants. Alt. Med. Rev. 1997, 2, 94–103. [Google Scholar]
- Kumar, S.; Kamboj, J.; Suman; Sharma, S. Overview for various aspects of the health benefits of Piper longum linn. fruit. J. Acupunct. Meridian Stud. 2011, 4, 134–140. [Google Scholar] [CrossRef]
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Antidiarrhoeal activity of piperine in mice. Planta Med. 2001, 67, 284–287. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Gajdzik, L.; Haberl, I.; Kraft, D.; Scheiner, O.; Graf, J. Hot spices influence permeability of human intestinal epithelial monolayers. J. Nutr. 1998, 128, 577–581. [Google Scholar]
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Piperine inhibits gastric emptying and gastrointestinal transit in rats and mice. Planta Med. 2001, 67, 176–179. [Google Scholar] [CrossRef]
- Ali, A.M.; Alam, N.M.; Yeasmin, M.S.; Khan, A.M.; Sayeed, M.A.; Rao, V.B. Antimicrobial screenings of different extracts of Piper longum Linn. Res. J. Agr. Bio. Sci. 2007, 3, 852–857. [Google Scholar]
- Kondo, S.; Sattaponpan, C.; Phongpaichit, S.; Srijan, A.; Itharat, A. Antibacterial activity of Thai medicinal plants Pikutbenjakul. J. Med. Assoc. Thai. 2010, 93 Suppl 7, S131–S135. [Google Scholar]
- Mehmood, M.H.; Gilani, A.H. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J. Med. Food 2010, 13, 1086–1096. [Google Scholar] [CrossRef]
- Dillinger, T.L.; Barriga, P.; Escarcega, S.; Jimenez, M.; Salazar Lowe, D.; Grivetti, L.E. Food of the gods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar]
- Schuier, M.; Sies, H.; Illek, B.; Fischer, H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. J. Nutr. 2005, 135, 2320–2325. [Google Scholar]
- Velazquez, C.; Calzada, F.; Esquivel, B.; Barbosa, E.; Calzada, S. Antisecretory activity from the flowers of Chiranthodendron pentadactylon and its flavonoids on intestinal fluid accumulation induced by Vibrio cholerae toxin in rats. J. Ethnopharmacol. 2009, 126, 455–458. [Google Scholar]
- Velazquez, C.; Correa-Basurto, J.; Garcia-Hernandez, N.; Barbosa, E.; Tesoro-Cruz, E.; Calzada, S.; Calzada, F. Anti-diarrheal activity of (−)-epicatechin from Chiranthodendron pentadactylon Larreat: Experimental and computational studies. J. Ethnopharmacol. 2012, 143, 716–719. [Google Scholar] [CrossRef]
- Velazquez, C.; Calzada, F.; Torres, J.; Gonzalez, F.; Ceballos, G. Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J. Ethnopharmacol. 2006, 103, 66–70. [Google Scholar] [CrossRef]
- Hor, M.; Rimpler, H.; Heinrich, M. Inhibition of intestinal chloride secretion by proanthocyanidins from Guazuma ulmifolia. Planta Med. 1995, 61, 208–212. [Google Scholar] [CrossRef]
- Chen, J.C.; Chang, Y.S.; Wu, S.L.; Chao, D.C.; Chang, C.S.; Li, C.C.; Ho, T.Y.; Hsiang, C.Y. Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa. J. Ethnopharmacol. 2007, 113, 233–239. [Google Scholar] [CrossRef]
- Becker, P.M.; Widjaja-Greefkes, H.C.; van Wikselaar, P.G. Inhibition of binding of the AB5-type enterotoxins LT-I and cholera toxin to ganglioside GM1 by galactose-rich dietary components. Foodborne Pathog. Dis. 2010, 7, 225–233. [Google Scholar] [CrossRef]
- Goncalves, C.; Berthiaume, F.; Mourez, M.; Dubreuil, J.D. Escherichia coli STb toxin binding to sulfatide and its inhibition by carragenan. FEMS Microbiol. Lett. 2008, 281, 30–35. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dubreuil, J.D. Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli. Toxins 2013, 5, 2009-2041. https://doi.org/10.3390/toxins5112009
Dubreuil JD. Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli. Toxins. 2013; 5(11):2009-2041. https://doi.org/10.3390/toxins5112009
Chicago/Turabian StyleDubreuil, J. Daniel. 2013. "Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli" Toxins 5, no. 11: 2009-2041. https://doi.org/10.3390/toxins5112009
APA StyleDubreuil, J. D. (2013). Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli. Toxins, 5(11), 2009-2041. https://doi.org/10.3390/toxins5112009