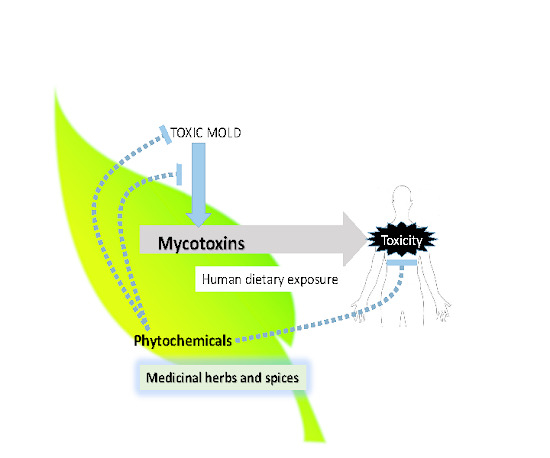
Nation-Based Occurrence and Endogenous Biological Reduction of Mycotoxins in Medicinal Herbs and Spices
Abstract
:1. Introduction
2. The Global Occurrence of Mycotoxins in Medicinal Herbs and Spices
| Country of Origin | Sample Name | Type of Mycotoxin | Maximum Concentration of Mycotoxin (μg/kg) | Reference |
|---|---|---|---|---|
| India | Asparagus racemosus | AFB1 | 220 | [7] |
| AFB2 | 50 | |||
| ZEA | 100 | |||
| Celery | AFB1 | 200 | ||
| ZEA | 70 | |||
| Cinnamomum zeylanicum | AFB1 | 140 | ||
| Cuminum cyminum | AFB1 | 310 | ||
| ZEA | 100 | |||
| Elettaria cardamomum | AFB1 | 400 | ||
| AFB2 | 210 | |||
| OTA | 50 | |||
| ZEA | 20 | |||
| Emblica officinalis | AFB1 | 380 | ||
| AFB2 | 80 | |||
| AFG1 | 170 | |||
| OTA | 120 | |||
| ZEA | 190 | |||
| Mesua ferrea | AFB1 | 270 | ||
| AFB2 | 100 | |||
| Long pepper | AFB1 | 570 | ||
| AFB2 | 160 | |||
| AFG1 | 190 | |||
| OTA | 80 | |||
| ZEA | 50 | |||
| Black pepper | AFB1 | 510 | ||
| AFB2 | 150 | |||
| OTA | 200 | |||
| ZEA | 100 | |||
| Indica | AFB1 | 310 | ||
| Baccala | AFB1 | 190 | ||
| Ginger | AFB1 | 370 | ||
| AFB2 | 220 | |||
| AFG1 | 100 | |||
| ZEA | 70 | |||
| Black cumin | AFB1 | 30 | [8] | |
| OTA | 35 | |||
| Fennel | AFB1 | 160 | ||
| OTA | 80 | |||
| Lime tree | AFB1 | 75 | ||
| Wormwood | AFB1 | 25 | ||
| OTA | 20 | |||
| Cinnamon | - | - | ||
| Peppermint | AFB1 | 25 | ||
| Carob tree | AFB1 | 10 | ||
| Chamomile | AFB1 | 145 | ||
| Saffron | - | - | ||
| Curcuma longa | - | - | ||
| Worm wood | AFB1 | 90 | ||
| China | Rhizoma coptidis-1 | OTA | 0.4 | [9] |
| Rhubarb | OTA | 0.2 | ||
| Ephedra | OTA | 0.3 | ||
| OTB | 0.4 | |||
| Fructus mume | OTA | 1.5 | ||
| OTB | 0.8 | |||
| Baohe pills | DON | 50.5 | [16] | |
| Sping Jujuba seed | AFB1 | 4.67 | [10] | |
| AFB2 | 0.89 | |||
| AFG1 | 2.14 | |||
| Barley | AFB1 | 1.72 | ||
| AFB2 | 0.95 | |||
| Areca seeds | AFB1 | 32.03 | ||
| AFB2 | 2.73 | |||
| AFG1 | 15.89 | |||
| Biota seed | AFB1 | 25.33 | ||
| AFB2 | 7.71 | |||
| AFG1 | 0.59 | |||
| AFG2 | 0.21 | |||
| Cassia seed | AFB1 | 5.69 | ||
| AFB2 | 1.81 | |||
| Nutmeg | AFB1 | 239.62 | ||
| AFB2 | 13.5 | |||
| AFG1 | 34.21 | |||
| AFG2 | 3.5 | |||
| Bitter orange | AFB1 | 0.15 | ||
| AFB2 | 0.77 | |||
| Pharbitis seed | AFB1 | 0.47 | ||
| Bitter apricot seed | AFB1 | 0.14 | ||
| AFB2 | 0.07 | |||
| AFG1 | 0.08 | |||
| AFG2 | 0.09 | |||
| White Aractylodes rhizome | AFB1 | 0.47 | ||
| AFB2 | 0.06 | |||
| Groomwell root | AFB1 | 1.03 | ||
| AFB2 | 0.48 | |||
| Japanese knotweed rhizome | AFB1 | 0.77 | ||
| AFB2 | 0.32 | |||
| Aractylodes rhizome | AFB1 | 0.58 | ||
| AFB2 | 0.93 | |||
| Corydalis rhizome | AFB1 | 68.4 | ||
| AFB2 | 1.71 | |||
| AFG1 | 0.95 | |||
| Coix seeds | AFB1 | 0.09 | ||
| AFB2 | 0.05 | |||
| ZEA | 211.4 | [17] | ||
| South Africa | Uthuvana | FB1 | 40 | [11] |
| Isica Katha | FB1 | 87 | ||
| Umsila Wengwe | FB1 | 117 | ||
| Sibindi | FB1 | 30 | ||
| Mudhora | FB1 | 25 | ||
| Matunga | FB1 | 139 | ||
| Mredeni | FB1 | 21 | ||
| Red carrot | FB1 | 30 | ||
| Roselina | FB1 | 126 | ||
| Seloka | FB1 | 67 | ||
| Thepe | FB1 | 26 | ||
| Saudi Arabia | Anise | AFB1, AFB2 | 38 | [18] |
| Black cumin | AFB1, AFB2 | 35 | ||
| Black pepper | ST | 40 | ||
| Red pepper | AFB1, AFB2 | 25 | ||
| Peppermint | AFB1, AFB2 | 17 | ||
| Cumin | ST | 20 | ||
| Marjoram | AFB1, AFB2 | 12 | ||
| Cinnamon | AFs | 4.67 | [19] | |
| Morocco | Pepper | AFs | 0.55 | [20] |
| Cumin | AFs | 0.18 | ||
| Ginger | AFs | 9.10 | ||
| Red paprika | AFs | 9.68 | ||
| USA | Ginger | AFs | 31 | [21] |
| Ginseng products | AFs | 0.1 | ||
| OTA | 10 | |||
| Ginseng root | AFs | 16 | [22] | |
| Kava-kava | AFB1 | 0.5 | [23] | |
| Milk thistle | AFs | 2.0 | [24] | |
| Spain | Sage leaves | AFs | 25.2 | [25] |
| OTA | 17.3 | |||
| FBs | 133.3 | |||
| DON | 102.2 | |||
| Citrinin | 273.2 | |||
| Chamomile flower | AFs | 161 | ||
| FBs | 90.0 | |||
| ZEA | 12.5 | |||
| DON | 191.5 | |||
| Citrinin | 51.6 | |||
| Valerian root | AFs | 15.8 | ||
| FBs | 96.7 | |||
| T2 | 13.3 | |||
| DON | 64.7 | |||
| Citrinin | 20.5 | |||
| Senna leaves | AFs | 434.3 | ||
| FBs | 86.7 | |||
| DON | 35.2 | |||
| Citrinin | 68.6 | |||
| Rhubarb | AFs | 71.2 | ||
| OTA | 13.9 | |||
| ZEA | 24.4 | |||
| T2 | 23.0 | |||
| DON | 58.4 | |||
| Citrinin | 42.9 | |||
| Artichoke | AFs | 12.1 | ||
| T2 | 29.8 | |||
| DON | 200.2 | |||
| Citrinin | 29.8 | |||
| Boldus | AFs | 86.6 | ||
| ZEA | 10.3 | |||
| T2 | 26.7 | |||
| DON | 343.5 | |||
| Citrinin | 25.8 | |||
| Burdock root | AFs | 10.3 | ||
| ZEA | 10.9 | |||
| Citrinin | 25.8 | |||
| Dandelion | AFs | 21.7 | ||
| OTA | 10.6 | |||
| ZEA | 17.0 | |||
| DON | 66.5 | |||
| Citrinin | 96.0 | |||
| Frangula | AFs | 64.7 | ||
| ZEA | 44.1 | |||
| T2 | 12.6 | |||
| DON | 60.9 | |||
| Citrinin | 38.4 | |||
| Ginkgo | AFs | 23.3 | ||
| T2 | 29.4 | |||
| DON | 134 | |||
| Citrinin | 354.8 | |||
| Lemon verbena | AFs | 37.7 | ||
| ZEA | 14.0 | |||
| T2 | 28.6 | |||
| DON | 143.7 | |||
| Citrinin | 79.1 | |||
| Olive leaves | AFs | 77.6 | ||
| ZEA | 42.7 | |||
| DON | 149.9 | |||
| Citrinin | 14.9 | |||
| Red tea | AFs | 853.4 | ||
| ZEA | 11.2 | |||
| T2 | 42.8 | |||
| DON | 179.9 | |||
| Citrinin | 22.3 | |||
| Ribgrass | AFs | 16.1 | ||
| T2 | 256.9 | |||
| Spearmint | AFs | 29.7 | ||
| DON | 91.1 | |||
| Citrinin | 43.3 | |||
| St Mary’s thistle | AFs | 11.5 | ||
| FBs | 236.7 | |||
| T2 | 35.6 | |||
| Star anise | AFs | 104.2 | ||
| FBs | 146.7 | |||
| ZEA | 10.1 | |||
| T2 | 60.5 | |||
| DON | 321.2 | |||
| Vervain | AFs | 104.5 | ||
| T2 | 20.4 | |||
| DON | 60.0 | |||
| Citrinin | 31.2 | |||
| White tea | AFs | 254.0 | ||
| ZEA | 11.2 | |||
| T2 | 42.8 | |||
| DON | 259.1 | |||
| Citrinin | 19.7 | |||
| Red paprika | OTA | 73.8 | [26] | |
| Licorice | OTA | 252.8 | [27] | |
| Turkey | Chamomile | AFB1 | 38.9 | [15] |
| Rose hip | AFB1 | 52.5 | ||
| Dried figs | AFs | 278.04 | [28] | |
| OTA | 15.31 | [29] | ||
| FB1 | 3649 | [14] |
3. Regulation of Fungal Growth and Mycotoxin Production by Components from Medicinal Herbs and Spices
4. Regulation of Mammalian Toxicity of Mycotoxins by Components from Medicinal Herbs and Spices
| Types of inhibition | Herbs and Spices | Effects on mycotoxicosis | References |
|---|---|---|---|
| fInhibition of fungal growth | Ajowain | A. flavus, A. parasiticus | [33] |
| Basil | A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [40] | |
| Cloves | Aspergillus, Penicillium | [35,36] | |
| A. flavus, A. parasiticus | [33] | ||
| Clove oil | A. flavus, A. parasiticus | [33] | |
| Cinnamon | Aspergillus, Penicillium | [35,36] | |
| A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [40] | ||
| A. flavus, A. parasiticus | [33] | ||
| Chinese cassia | Aspergillus, Penicillium | [35] | |
| Coriander | A. flavus, A. parasiticus | [33] | |
| Kalonji | A. flavus, A. parasiticus | [33] | |
| Kalonji oil | A. flavus, A. parasiticus | [33] | |
| Marigold | A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [31] | |
| Neem oil | A. flavus, A. parasiticus | [33] | |
| Quyssum | A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [40] | |
| Spearmint | A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [40] | |
| Thyme | Aspergillus, Penicillium | [35] | |
| A. flavus, A. parasiticus, A. ochraceus, A. fumigatus, Fusarium spp. | [42,49] | ||
| Thyme oil | A. flavus, A. parasiticus, A. ochraceus, F. moniliforme | [40] | |
| Turmeric | A. flavus, A. parasiticus | [33] | |
| Inhibition of mycotoxin production | Anise | Sterigmatocystin, citrinin | [18] |
| Black cumin | AFB, sterigmatocystin, citrinin | ||
| Black pepper | AF, sterigmatocystin | ||
| Peppermint | AFB1, citrinin | ||
| Cardamom | AFB1, sterigmatocystin, citrinin | ||
| Clove | AF, sterigmatocystin, citrinin | ||
| Cumin | AFB1, citrinin | ||
| Ginger | Sterigmatocystin | ||
| Marjoram | AFB1, citrinin | ||
| Sweet basil leaves | AFB1 | [39] | |
| Inhibition of mycotoxin actionn | Caffeic acid phenethyl ester | AFB1 | [49,59] |
| Normalization of γGT, ALP, GST and NO | |||
| Catechin | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Chitosan | AFB1 | [50] | |
| Normalization of AST and ALT levels | |||
| Chlorogenic acid | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Turmeric | AFB1 | [47,48] | |
| Normalization of LDH and ALT | |||
| Cyanidin | AFB1, OTA | [52,53,54,55] | |
| Normalization of ROS, protein and DNA synthesis, and apoptosis in HepG2 and Caco-2 cells | |||
| Diallyl sulfide | AFB1 | [61] | |
| Reduction of DNA damage | |||
| Epigallocatechin-3-gallate | Deoxynivalenol, HT-2 toxin | [73,74] | |
| Suppression of inflammatory responses | |||
| Eugenol | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Fisetin | AFB1 | [59,67] | |
| Prevention of carcinogenesis Attenuation of DNA adduct formation | |||
| Genistein | AFB1 | [62] | |
| Reduction of mutagenesis | |||
| Indole-3-carbinol | AFB1 | [77] | |
| Prevention of carcinogenesis in rat liver | |||
| Kaempferol | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Lycopene | AFB1, OTA ZEA | [56,57,58,75,76] | |
| Protection effect on oxidative, inflammatory, endocrine and reproductive damage in mice | |||
| Morin | AFB1 | [59,67] | |
| Prevention of carcinogenesis Attenuation of DNA adduct formation | |||
| Naringin | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Quercetin | AFB1 | [67] | |
| Prevention of carcinogenesis | |||
| Robinetin | AFB1 | [67] | |
| Prevention of carcinogenesis | |||
| Sulforaphane | AFB1 | [63,64,65] | |
| Induction of hepatic total GST activity. Attenuation of DNA adduct formation | |||
| Thyme oil | AFB1 | [46,66] | |
| Excretion of AFs Normalization of AST, ALP and γGT Ameliorative effect on oxidative stress and genotoxicity | |||
| Vanillin | AFB1 | [59] | |
| Attenuation of DNA adduct formation | |||
| Gingerol | Patulin | [37] | |
| Reduction of DNA damage in HepG2 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kosalec, I.; Cvek, J.; Tomic, S. Contaminants of medicinal herbs and herbal products. Arh. Hig. Rada Toksikol. 2009, 60, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Watson, L.; Ernst, E. Contamination and adulteration of herbal medicinal products (HMPs): An overview of systematic reviews. Eur. J. Clin. Pharmacol. 2013, 69, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, H.; Xu, W.; Chen, D.; Yu, J.; Li, J.; Li, L. Fecal metabolome profiling of liver cirrhosis and hepatocellular carcinoma patients by ultra performance liquid chromatography-mass spectrometry. Anal. Chim. Acta 2011, 691, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bucci, T.J.; Howard, P.C.; Tolleson, W.H.; Laborde, J.B.; Hansen, D.K. Renal effects of fumonisin mycotoxins in animals. Toxicol. Pathol. 1998, 26, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, E.; Ziegler, K. Ochratoxin a from a toxicological perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Razzazi-Fazeli, E.; Porasuphatana, S.; Bohm, J. Contamination of aflatoxins in herbal medicinal products in thailand. Mycopathologia 2004, 158, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, H.K. Mycobiota and mycotoxins in herbal drugs of indian pharmaceutical industries in india. Mycol. Res. 1995, 99, 697–703. [Google Scholar] [CrossRef]
- Aziz, N.H.; Youssef, Y.A.; El-Fouly, M.Z.; Moussa, L.A. Contamination of some medicinal plant samples and spices by fungi and their mycotoxins. Bot. Bull. Acad. Sin. 1998, 39, 279–285. [Google Scholar]
- Han, Z.; Zheng, Y.; Luan, L.; Ren, Y.; Wu, Y. Analysis of ochratoxin A and ochratoxin B in traditional chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry using [13C20]-ochratoxin A as an internal standard. J. Chromatogr. A 2010, 1217, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, H.; Sun, L.; Ma, S.; Lin, R. Determination of aflatoxins in medicinal herbs by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 2012, 23, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Katerere, D.R.; Stockenstrom, S.; Thembo, K.M.; Rheeder, J.P.; Shephard, G.S.; Vismer, H.F. A preliminary survey of mycological and fumonisin and aflatoxin contamination of african traditional herbal medicines sold in south africa. Hum. Exp. Toxicol. 2008, 27, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Brera, C.; Elakhdari, S.; Catano, C.; Debegnach, F.; Angelini, S.; de Santis, B.; Faid, M.; Benlemlih, M.; Minardi, V.; et al. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control 2006, 17, 868–874. [Google Scholar] [CrossRef]
- Boyacioglu, D.; Gonul, M. Survey of aflatoxin contamination of dried figs grown in Turkey in 1986. Food Addit. Contam. 1990, 7, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Karbancioglu-Guler, F.; Heperkan, D. Natural occurrence of fumonisin B1 in dried figs as an unexpected hazard. Food Chem. Toxicol. 2009, 47, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Arino, A.; Herrera, M.; Estopanan, G.; Juan, T. High levels of ochratoxin A in licorice and derived products. Int. J. Food Microbiol. 2007, 114, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, X.; Pan, J.; Ou-Yang, Z.; Wu, J.; Yang, M. Determination of deoxynivalenol in medicinal herbs and related products by GC–ECD and confirmation by GC–MS. Chromatographia 2010, 71, 533–538. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Logrieco, A.F.; Yang, M.; Ou-Yang, Z.; Wang, X.; Guo, Q. Determination of zearalenone in traditional Chinese medicinal plants and related products by HPLC-FLD. J. Food Sci. 2011, 28, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, F.M. Spices mycobiota and mycotoxins available in saudi arabia and their abilities to inhibit growth of some toxigenic fungi. Mycobiology 2007, 35, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Al-juraifani, A.A. Natural occurrence of fungi and aflatoxins of cinnamon in the Saudi Arabia. Afr. J. Food Sci. 2011, 5, 460–465. [Google Scholar]
- Zinedine, A.; Mañes, J. Occurrence and legislation of mycotoxins in food and feed from Morocco. Food Control 2009, 20, 334–344. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Weaver, C.M.; Oles, C.J.; Rump, L.V.; White, K.D.; Betz, J.M.; Rader, J.I. Use of multitoxin immunoaffinity columns for determination of aflatoxins and ochratoxin A in ginseng and ginger. J. AOAC Int. 2007, 90, 1042–1049. [Google Scholar] [PubMed]
- D’Ovidio, K.; Trucksess, M.; Weaver, C.; Horn, E.; McIntosh, M.; Bean, G. Aflatoxins in ginseng roots. Food Addit. Contam. 2006, 23, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Trucksess, M.W. Determination of aflatoxins in botanical roots by a modification of AOAC Official Method 991.31: Single-laboratory validation. J. AOAC Int. 2010, 93, 184–189. [Google Scholar] [PubMed]
- Tournas, V.H.; Sapp, C.; Trucksess, M.W. Occurrence of aflatoxins in milk thistle herbal supplements. Food Addit. Contam. 2012, 29, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Hernandez Hierro, J.M.; Garcia-Villanova, R.J.; Rodriguez Torrero, P.; Toruno Fonseca, I.M. Retail sale in Spain: Occurrence and evaluation of a simultaneous analytical method. J. Agric. Food Chem. 2008, 56, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Tosun, H.; Arslan, R. Determination of aflatoxin B1 levels in organic spices and herbs. Scientific World J. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bircan, C.; Koç, M. Aflatoxins in dried figs in Turkey: A comparative survey on the exported and locally consumed dried figs for assessment of exposure. J. Agric. Sci. 2012, 14, 1265–1274. [Google Scholar]
- Karbancioglu-Guler, F.; Heperkan, D. Natural occurrence of ochratoxin A in dried figs. Anal. Chim. Acta 2008, 617, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; An, T.J.; Kim, J.; Park, S.H.; Kim, D.; Ahn, Y.S.; Moon, Y. Postharvest strategies for deoxynivalenol and zearalenone reduction in stored adlay (Coix lachryma-jobi L.) grains. J. Food Prot. 2014, 77, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Juglal, S.; Govinden, R.; Odhav, B. Spice oils for the control of co-occurring mycotoxin-producing fungi. J. Food Prot. 2002, 65, 683–687. [Google Scholar] [PubMed]
- Paranagama, P.A.; Abeysekera, K.H.; Abeywickrama, K.; Nugaliyadde, L. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 2003, 37, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Shafqatullah; Ali, J.; Zia-ur-Rehman. Inhibition of aflatoxin producing fungus growth using chemical, herbal compounds/spices and plants. Pure Appl. Biol. 2012, 1, 8–13. [Google Scholar]
- Azzouz, M.A. The Inhibitory Effects of Herbs, Spices and Other Plant Materials on Mycotoxigenic Moulds. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 1 January 1981. [Google Scholar]
- Mostafa, E.M. Mycoflora and Mycotoxins of Some Spices. Master Thesis, Botany Dept., Faculty of Science, Assiut University, Assiut, Egypt, 10 June 1990. [Google Scholar]
- Hitokoto, H.; Morozumi, S.; Wauke, T.; Sakai, S.; Kurata, H. Inhibitory effects of spices on growth and toxin production of toxigenic fungi. Appl. Environ. Microbiol. 1980, 39, 818–822. [Google Scholar] [PubMed]
- Mabrouk, S.S.; El-Shayeb, N.M. Inhibition of aflatoxin formation by some spices. Z. Lebensm. Unters. Forsch. 1980, 171, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, M.S.; Bhat, R.V. Aspergillus parasiticus growth and aflatoxin production on black and white pepper and the inhibitory action of their chemical constituents. Appl. Environ. Microbiol. 1984, 48, 376–379. [Google Scholar] [PubMed]
- Atanda, O.O.; Akpan, I.; Oluwafemi, F. The potential of some spice essential oils in the control of A. Parasiticus CFR 223 and aflatoxin production. Food Control 2007, 18, 601–607. [Google Scholar] [CrossRef]
- Soliman, K.M.; Badeaa, R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Montes-Belmont, R.; Carvajal, M. Control of aspergillus flavus in maize with plant essential oils and their components. J. Food Prot. 1998, 61, 616–619. [Google Scholar] [PubMed]
- Basilico, M.Z.; Basilico, J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B. Inhibition of aflatoxin production by cinnamon. J. Food Sci. 1974, 39, 1163–1165. [Google Scholar] [CrossRef]
- Morozumi, S. Isolation, purification, and antibiotic activity of o-methoxycinnamaldehyde from cinnamon. Appl. Environ. Microbiol. 1978, 36, 577–583. [Google Scholar] [PubMed]
- Dwividi, S.A.; Dubey, B.L. Potentionial use of essential oil of the trachyepermum ammy against seed borne fungi of guar (Cyamopsis tetragonoloba L.). Mycopathologia 1993, 121, 101–104. [Google Scholar] [CrossRef]
- Abdel-Fattah, S.M.; Abosrea, Y.H.; Shehata, F.E.; Flourage, M.R.; Helal, A.D. The efficacy of thyme oil as antitoxicant of aflatoxin(s) toxicity in sheep. J. Am. Sci. 2010, 6, 948–960. [Google Scholar]
- Nayak, S.; Sashidhar, R.B. Metabolic intervention of aflatoxin B1 toxicity by curcumin. J. Ethnopharmacol. 2010, 127, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Gowda, N.K.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 2008, 87, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Akcam, M.; Artan, R.; Yilmaz, A.; Ozdem, S.; Gelen, T.; Naziroglu, M. Caffeic acid phenethyl ester modulates aflatoxin B1-induced hepatotoxicity in rats. Cell Biochem. Funct. 2013, 31, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhaba, M.A.; Aljawish, A.; El-Nekeety, A.A.; Abdel-Aiezm, S.H.; Abdel-Kader, H.A.M.; Rihn, B.H.; Joubert, O. Chitosan nanoparticles and quercetin modulate geneexpression and prevent the genotoxicity of aflatoxinB1in rat liver. Toxicol. Rep. 2015, 2, 737–747. [Google Scholar] [CrossRef]
- Subhapradha, N.; Saravanan, R.; Ramasamy, P.; Srinivasan, A.; Shanmugam, V.; Shanmugam, A. Hepatoprotective effect of β-Chitosan from Gladius of Sepioteuthis lessoniana against carbontetrachloride-induced oxidative stress in Wistar rats. Appl. Biochem. Biotechnol. 2014, 172, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Bognanno, M.; Grilli, E.; D’Orazio, N.; Galvano, F. Dimethylarginine dimethylaminohydrolase/nitric oxide synthase pathway in liver and kidney: Protective effect of cyanidin 3-O-β-d-glucoside on ochratoxin-A toxicity. Toxins 2012, 4, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, C.; Acquaviva, R.; Piva, A.; Sorrenti, V.; Vanella, L.; Piva, G.; Casadei, G.; la Fauci, L.; Ritieni, A.; Bognanno, M.; et al. Protective effect of cyanidin 3-O-β-d-glucoside on ochratoxin A-mediated damage in the rat. Br. J. Nutr. 2007, 98, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; La Fauci, L.; Acquaviva, R.; Campisi, A.; Raciti, G.; Scifo, C.; Renis, M.; Galvano, G.; Vanella, A.; Galvano, F. Ochratoxin A-induced DNA damage in human fibroblast: Protective effect of cyanidin 3-O-β-d-glucoside. J. Nutr. Biochem. 2005, 16, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.C.; Galvano, F.; Bonsi, L.; Speroni, E.; Costa, S.; Renzulli, C.; Cervellati, R. Cyanidin-3-O-β-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br. J. Nutr. 2005, 94, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Palabiyik, S.S.; Erkekoglu, P.; Sahin, G.; Basaran, N.; Giray, B.K. The carotenoid lycopene protects rats against DNA damage induced by ochratoxin A. Toxicon 2013, 73, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, S.S.; Erkekoglu, P.; Zeybek, N.D.; Kizilgun, M.; Baydar, D.E.; Sahin, G.; Giray, B.K. Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp. Toxicol. Pathol. 2013, 65, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Aboobaker, V.S.; Balgi, A.D.; Bhattacharya, R.K. In vivo effect of dietary factors on the molecular action of aflatoxin B1: Role of non-nutrient phenolic compounds on the catalytic activity of liver fractions. In Vivo 1994, 8, 1095–1098. [Google Scholar] [PubMed]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical biochemistry of hepatotoxicity. J. Clin. Toxicol. 2011. [Google Scholar] [CrossRef]
- Sheen, L.Y.; Wu, C.C.; Lii, C.K.; Tsai, S.J. Effect of diallyl sulfide and diallyl disulfide, the active principles of garlic, on the aflatoxin B1-induced DNA damage in primary rat hepatocytes. Toxicol. Lett. 2001, 122, 45–52. [Google Scholar] [CrossRef]
- Polivkova, Z.; Langova, M.; Smerak, P.; Bartova, J.; Barta, I. Antimutagenic effect of genistein. Czech J. Food Sci. 2006, 24, 119–126. [Google Scholar]
- Gross-Steinmeyer, K.; Stapleton, P.L.; Tracy, J.H.; Bammler, T.K.; Strom, S.C.; Eaton, D.L. Sulforaphane- and phenethyl isothiocyanate-induced inhibition of aflatoxin B1-mediated genotoxicity in human hepatocytes: Role of GSTM1 genotype and CYP3A4 gene expression. Toxicol. Sci. 2010, 116, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.L.; Egner, P.A.; Wiriyachan, N.; Ruchirawat, M.; Kensler, K.H.; Wogan, G.N.; Groopman, J.D.; Croy, R.G.; Essigmann, J.M. Sulforaphane-mediated reduction of aflatoxin B1-N7-guanine in rat liver DNA: Impacts of strain and sex. Toxicol. Sci. 2011, 121, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Chen, X.Y.; Zhu, R.Z.; Choi, B.M.; Kim, B.R. Sulforaphane induces glutathione S-transferase isozymes which detoxify aflatoxin B1-8,9-epoxide in AML 12 cells. BioFactors 2010, 36, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziem, S.H.; Hassan, A.M.; El-Denshary, E.S.; Hamzawy, M.A.; Mannaa, F.A.; Abdel-Wahhab, M.A. Ameliorative effects of thyme and calendula extracts alone or in combination against aflatoxins-induced oxidative stress and genotoxicity in rat liver. Cytotechnology 2014, 66, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.K.; Firozi, P.F. Effect of plant flavonoids on microsome catalyzed reactions of aflatoxin B1 leading to activation and DNA adduct formation. Cancer Lett. 1988, 39, 85–91. [Google Scholar] [CrossRef]
- Raghubeer, S.; Naqiah, S.; Phulukdaree, A.; Chuturgoon, A. The phytoalexin resveratrol ameliorates ochratoxin A toxicity in human embryonic kidney (HEK293) cells. J. Cell Biochem. 2015, in press. [Google Scholar]
- Sridhar, M.; Suganthi, R.U.; Thammiaha, V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. Anim. Nutr. 2014. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch. Toxicol. 2010, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Gonzalez-Arias, C.A.; Ramos, A.J.; Sanchis, V.; Fernandez-Cruz, M.L. Cytotoxicity of the mycotoxins deoxynivalenol and ochratoxin A on CaCo-2 cell line in presence of resveratrol. Toxicol. Vitro 2015, 29, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhong, L.; Jiang, L.; Geng, C.; Cao, J.; Sun, X.; Liu, X.; Chen, M.; Ma, Y. 6-gingerol prevents patulin-induced genotoxicity in HepG2 cells. Phytother. Res. 2011, 25, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, P.; Rajashree, K.; Bharathi Priya, L.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kinoshita, M.; Kamata, Y.; Minai, Y.; Sugita-Konishi, Y. (−)-Epigallocatechin gallate suppresses the cytotoxicity induced by trichothecene mycotoxins in mouse cultural macrophages. Mycotoxin Res. 2011, 27, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Funck, V.R.; Borges Filho, C.; del Fabbro, L.; de Gomes, M.G.; Donato, F.; Royes, L.F.; Oliveira, M.S.; Jesse, C.R.; Furian, A.F. Lycopene protects against acute zearalenone-induced oxidative, endocrine, inflammatory and reproductive damages in male mice. Chem. Biol. Interact. 2015, 230, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Filho, C.B.; Del’Fabbro, L.; Roman, S.S.; Royes, L.F.; Fighera, M.R.; Jesse, C.R.; Oliveira, M.S.; Furian, A.F. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male swiss mice. Exp. Toxicol. Pathol. 2014, 66, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M.; Hudson, E.A.; Ball, H.W.; Barrett, M.C.; Clark, H.L.; Judah, D.J.; Verschoyle, R.D.; Neal, G.E. Chemoprevention of aflatoxin B1-induced carcinogenesis by indole-3-carbinol in rat liver—Predicting the outcome using early biomarkers. Carcinogenesis 1998, 19, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, K.H.; An, T.J.; Oh, S.-K.; Moon, Y. Nation-Based Occurrence and Endogenous Biological Reduction of Mycotoxins in Medicinal Herbs and Spices. Toxins 2015, 7, 4111-4130. https://doi.org/10.3390/toxins7104111
Do KH, An TJ, Oh S-K, Moon Y. Nation-Based Occurrence and Endogenous Biological Reduction of Mycotoxins in Medicinal Herbs and Spices. Toxins. 2015; 7(10):4111-4130. https://doi.org/10.3390/toxins7104111
Chicago/Turabian StyleDo, Kee Hun, Tae Jin An, Sang-Keun Oh, and Yuseok Moon. 2015. "Nation-Based Occurrence and Endogenous Biological Reduction of Mycotoxins in Medicinal Herbs and Spices" Toxins 7, no. 10: 4111-4130. https://doi.org/10.3390/toxins7104111
APA StyleDo, K. H., An, T. J., Oh, S. -K., & Moon, Y. (2015). Nation-Based Occurrence and Endogenous Biological Reduction of Mycotoxins in Medicinal Herbs and Spices. Toxins, 7(10), 4111-4130. https://doi.org/10.3390/toxins7104111