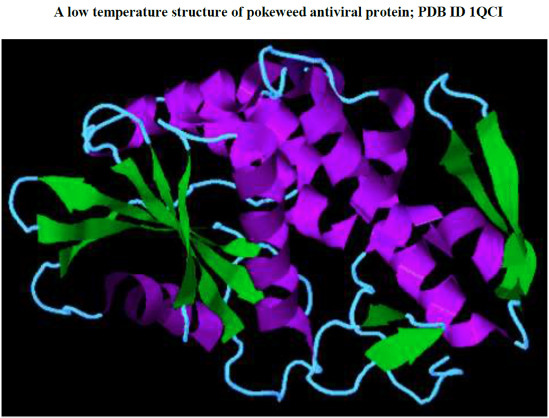
Pokeweed Antiviral Protein, a Ribosome Inactivating Protein: Activity, Inhibition and Prospects
Abstract
:1. Introduction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsnes, S. The history of ricin, abrin and related toxins. Toxicon 2004, 44, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.W.; Cieslak, T.J.; Pavlin, J.A.; Eitzen, E.M. Biological warfare. A historical perspective. JAMA 1997, 278, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Knight, B. Ricin—A potent homicidal poison. Br. Med. J. 1979, 1, 350–351. [Google Scholar] [PubMed]
- Wiener, S.L. Strategies for the prevention of a successful biological warfare aerosol attack. Mil. Med. 1996, 161, 251–256. [Google Scholar] [PubMed]
- Lodge, J.K.; Kaniewski, W.K.; Tumer, N.E. Broad-spectrum virus resistance in transgenic plants expressing pokeweed antiviral protein. Proc. Natl. Acad. Sci. USA 1993, 90, 7089–7093. [Google Scholar] [CrossRef] [PubMed]
- Jach, G.; Gornhardt, B.; Mundy, J.; Logemann, J.; Pinsdorf, E.; Leah, R.; Schell, J.; Maas, C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; FitzGerald, D.; Siegall, C.; Press, O.W. Advances in immunotoxin biology and therapy: A summary of the fourth international symposium on immunotoxins. Cancer Res. 1996, 56, 926–932. [Google Scholar] [PubMed]
- Kreitman, R.J. Immunotoxins in cancer therapy. Curr. Opin. Immunol. 1999, 11, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; FitzGerald, D. Recombinant toxins for cancer treatment. Science 1991, 254, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Christie, A. House of lurking death. In Partners in Crime; Dodd, Mead and Company: New York, NY, USA, 1929. [Google Scholar]
- Peumans, W.J.; Hao, Q.; van Damme, E.J. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Irvin, J.D. Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Arch. Biochem. Biophys. 1975, 169, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Fordham-Skelton, A.P.; Taylor, P.N.; Hartley, M.R.; Croy, R.R. Characterisation of saporin genes: in vitro expression and ribosome inactivation. Mol. Gen. Genet. 1991, 229, 460–466. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Ready, M.P.; Brown, D.T.; Robertus, J.D. Extracellular localization of pokeweed antiviral protein. Proc. Natl. Acad. Sci. USA 1986, 83, 5053–5056. [Google Scholar] [CrossRef] [PubMed]
- Bonness, M.S.; Ready, M.P.; Irvin, J.D.; Mabry, T.J. Pokeweed antiviral protein inactivates pokeweed ribosomes; implications for the antiviral mechanism. Plant J. 1994, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Domashevskiy, A.V. Turnip Mosaic Virus Genome-Linked Protein (VPg) Inhibits Pokeweed Antiviral Protein (PAP)-Mediated Depurination of RNA. Ph.D. Thesis, Graduate Center and Hunter College, City University of New York, New York, NY, USA, May 2012. [Google Scholar]
- Ehrlich, P. Experimentelle Untersuchungenüber Immunität I. Ueber Ricin. Dtsch. Med. Wochenschr. 1891, 17, 976–979. (In German) [Google Scholar] [CrossRef]
- Ehrlich, P. Experimentelle Untersuchungenüber Immunität I. Ueber Abrin. Dtsch. Med. Wochenschr. 1891, 17, 1218–1219. (In German) [Google Scholar] [CrossRef]
- Lin, J.Y.; Liu, K.; Chen, C.C.; Tung, T.C. Effect of crystalline ricin on the biosynthesis of protein, RNA, and DNA in experimental tumor cells. Cancer Res. 1971, 31, 921–924. [Google Scholar] [PubMed]
- Montanaro, L.; Sperti, S.; Stirpe, F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem. J. 1973, 136, 677–683. [Google Scholar] [PubMed]
- Obrig, T.G.; Irvin, J.D.; Hardesty, B. The effect of an antiviral peptide on the ribosomal reactions of the peptide elongation enzymes, EF-I and EF-II. Arch. Biochem. Biophys. 1973, 155, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Mundy, J.; Leah, R.; Boston, R.; Endo, Y.; Stirpe, F. Genes encoding ribosome-inactivating proteins. Plant Mol. Biol. Rep. 1994, 12, S60–S62. [Google Scholar] [CrossRef]
- Girbes, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef] [PubMed]
- Husain, J.; Tickle, I.J.; Wood, S.P. Crystal structure of momordin, a type I ribosome inactivating protein from the seeds of Momordica charantia. FEBS Lett. 1994, 342, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Mlsna, D.; Monzingo, A.F.; Katzin, B.J.; Ernst, S.; Robertus, J.D. Structure of recombinant ricin A chain at 2.3 A. Protein Sci. 1993, 2, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Monzingo, A.F.; Robertus, J.D. X-ray analysis of substrate analogs in the ricin A-chain active site. J. Mol. Biol. 1992, 227, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Savino, C.; Federici, L.; Ippoliti, R.; Lendaro, E.; Tsernoglou, D. The crystal structure of saporin SO6 from Saponaria officinalis and its interaction with the ribosome. FEBS Lett. 2000, 470, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Monzingo, A.F.; Collins, E.J.; Ernst, S.R.; Irvin, J.D.; Robertus, J.D. The 2.5 A structure of pokeweed antiviral protein. J. Mol. Biol. 1993, 233, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Fu, Z.; Chen, M.; Lin, Y.; Pan, K. Structure of trichosanthin at 1.88 A resolution. Proteins 1994, 19, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hosur, M.V.; Nair, B.; Satyamurthy, P.; Misquith, S.; Surolia, A.; Kannan, K.K. X-ray structure of gelonin at 1.8 A resolution. J. Mol. Biol. 1995, 250, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Barbieri, L.; Girbes, T.; Battelli, M.G.; Rojo, M.A.; Arias, F.J.; Rocher, M.A.; Soriano, F.; Mendez, E.; Stirpe, F. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae). Biochim. Biophys. Acta. 1993, 1216, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kurinov, I.V.; Myers, D.E.; Irvin, J.D.; Uckun, F.M. X-ray crystallographic analysis of the structural basis for the interactions of pokeweed antiviral protein with its active site inhibitor and ribosomal RNA substrate analogs. Protein Sci. 1999, 8, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Pihl, A. Isolation and properties of abrin: A toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur. J. Biochem. 1973, 35, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Pihl, A. Chimeric toxins. Pharmacol. Ther. 1981, 15, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Gasperi-Campani, A.; Barbieri, L.; Lorenzoni, E.; Montanaro, L.; Sperti, S.; Bonetti, E. Inhibition of protein synthesis by modeccin, the toxin of Modecca digitata. FEBS Lett. 1977, 85, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Olsnes, S.; Pihl, A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 1976, 251, 3977–3984. [Google Scholar] [PubMed]
- Olsnes, S.; Sandvig, K. How protein toxins enter and kill cells. Cancer Treat. Res. 1988, 37, 39–73. [Google Scholar] [PubMed]
- Steeves, R.M.; Denton, M.E.; Barnard, F.C.; Henry, A.; Lambert, J.M. Identification of three oligosaccharide binding sites in ricin. Biochemistry 1999, 38, 11677–11685. [Google Scholar] [CrossRef] [PubMed]
- Van Deurs, B.; Tonnessen, T.I.; Petersen, O.W.; Sandvig, K.; Olsnes, S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J. Cell Biol. 1986, 102, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Beaumelle, B.; Alami, M.; Hopkins, C.R. ATP-dependent translocation of ricin across the membrane of purified endosomes. J. Biol. Chem. 1993, 268, 23661–23669. [Google Scholar] [PubMed]
- Sandvig, K.; van Deurs, B. Endocytosis and intracellular sorting of ricin and Shiga toxin. FEBS Lett. 1994, 346, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B.; Read, R.J. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 1997, 36, 11051–11054. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Roberts, L.M. Toxin entry: Retrograde transport through the secretory pathway. J. Cell Biol. 1998, 140, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; van Deurs, B. Endocytosis and intracellular transport of ricin: Recent discoveries. FEBS Lett. 1999, 452, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Kaloyanova, D.; Kyurkchiev, S.; Xu, J.; Abouhaidar, M.; Ivanov, I. Mouse monoclonal antibodies against Phytolacca americana antiviral protein PAP I. Hybridoma 1999, 18, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Parikh, B.A.; Baykal, U.; Di, R.; Tumer, N.E. Evidence for retro-translocation of pokeweed antiviral protein from endoplasmic reticulum into cytosol and separation of its activity on ribosomes from its activity on capped RNA. Biochemistry 2005, 44, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Bass, H.W.; Webster, C.; O’Brian, G.R.; Roberts, J.K.; Boston, R.S. A maize ribosome-inactivating protein is controlled by the transcriptional activator Opaque-2. Plant Cell 1992, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, B.; Muller-Uri, F.; Cameron-Mills, V.; Gough, S.; Simpson, D.; Skriver, K.; Mundy, J. The barley 60 kDa jasmonate-induced protein (JIP60) is a novel ribosome-inactivating protein. Plant J. 1994, 6, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, S.; Reinbothe, C.; Lehmann, J.; Becker, W.; Apel, K.; Parthier, B. JIP60, a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc. Natl. Acad. Sci. USA 1994, 91, 7012–7016. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.A.; Morgan, A.E.; Hey, T.D. Characterization and molecular cloning of a proenzyme form of a ribosome-inactivating protein from maize. Novel mechanism of proenzyme activation by proteolytic removal of a 2.8-kilodalton internal peptide segment. J. Biol. Chem. 1991, 266, 23422–23427. [Google Scholar] [PubMed]
- Jimenez, A.; Vazquez, D. Plant and fungal protein and glycoprotein toxins inhibiting eukaryote protein synthesis. Annu. Rev. Microbiol. 1985, 39, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Barbieri, L. Ribosome-inactivating proteins up to date. FEBS Lett. 1986, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Valbonesi, P.; Bondioli, M.; Alvarez, M.L.; Dal Monte, P.; Landini, M.P.; Stirpe, F. Adenine glycosylase activity in mammalian tissues: An equivalent of ribosome-inactivating proteins. FEBS Lett. 2001, 505, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Barbieri, L.; Gorini, P.; Valbonesi, P.; Bolognesi, A.; Polito, L. Activities associated with the presence of ribosome-inactivating proteins increase in senescent and stressed leaves. FEBS Lett. 1996, 382, 309–312. [Google Scholar] [CrossRef]
- Girbés, T.; de Torre, C.; Iglesias, R.; Ferreras, J.M.; Mendéz, E. RIP for viruses. Nature 1996, 379, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Rippmann, J.F.; Michalowski, C.B.; Nelson, D.E.; Bohnert, H.J. Induction of a ribosome-inactivating protein upon environmental stress. Plant Mol. Biol. 1997, 35, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Melton-Celsa, A.R. Shiga toxin (Stx) classification, structure, and function. Microbiol. Spectr. 2014, 9, 1–13. [Google Scholar]
- Russo, L.M.; Melton-Celsa, A.R.; Smith, M.J.; O’Brien, A.D. Comparisons of native shiga Toxins (Stxs) Type 1 and 2 with chimeric toxins indicate that the source of the binding subunit dictates degree of toxicity. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Ussery, M.A.; Leppla, S.H.; Rothman, S.W. Inhibition of protein synthesis by Shiga toxin: Activation of the toxin and inhibition of peptide elongation. FEBS Lett. 1980, 117, 84–88. [Google Scholar] [CrossRef]
- Sandvig, K.; van Deurs, B. Transport of protein toxins into cells: Pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002, 529, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bergan, J.; Dyve Lingelem, A.B.; Simm, R.; Skotland, T.; Sandvig, K. Shiga toxins. Toxicon 2012, 60, 1085–1107. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001, 393, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. Hypsin, a novel thermostable ribosome-inactivating protein with antifungal and antiproliferative activities from fruiting bodies of the edible mushroom Hypsizigus marmoreus. Biochem. Biophys. Res. Commun. 2001, 285, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.Z.; Yu, M.M.; Ooi, L.S.; Ng, T.B.; Chang, S.T.; Sun, S.S.; Ooi, V.E. Isolation and Characterization of a Type 1 Ribosome-Inactivating Protein from Fruiting Bodies of the Edible Mushroom (Volvariella volvacea). J. Agric. Food Chem. 1998, 46, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Yang, J.H.; Liu, W.Y. Isolation and enzymatic characterization of lamjapin, the first ribosome-inactivating protein from cryptogamic algal plant (Laminaria japonica A). Eur. J. Biochem. 2002, 269, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Chen, Z.C.; Antoniw, J.F.; White, R.F. Isolation and characterization of a cDNA clone encoding the anti-viral protein from Phytolacca americana. Plant Mol. Biol. 1991, 17, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Irvin, J.D.; Uckun, F.M. Pokeweed antiviral protein: Ribosome inactivation and therapeutic applications. Pharmacol. Ther. 1992, 55, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.E.; Irvin, J.D.; Smith, R.S.; Kuebelbeck, V.M.; Uckun, F.M. Production of a pokeweed antiviral protein (PAP)-containing immunotoxin, B43-PAP, directed against the CD19 human B lineage lymphoid differentiation antigen in highly purified form for human clinical trials. J. Immunol. Methods 1991, 136, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Poyet, J.L.; Radom, J.; Hoeveler, A. Isolation and characterization of a cDNA clone encoding the pokeweed antiviral protein II from Phytolacca americana and its expression in E. coli. FEBS Lett. 1994, 347, 268–272. [Google Scholar] [CrossRef]
- Rajamohan, F.; Engstrom, C.R.; Denton, T.J.; Engen, L.A.; Kourinov, I.; Uckun, F.M. High-level expression and purification of biologically active recombinant pokeweed antiviral protein. Protein Expr. Purif. 1999, 16, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Poyet, J.L.; Hoeveler, A. cDNA cloning and expression of pokeweed antiviral protein from seeds in Escherichia coli and its inhibition of protein synthesis in vitro. FEBS Lett. 1997, 406, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Aron, G.M.; Irvin, J.D.; Stirpe, F. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolacca americana L. (pokeweed). Biochem. J. 1982, 203, 55–59. [Google Scholar] [PubMed]
- Honjo, E.; Dong, D.; Motoshima, H.; Watanabe, K. Genomic clones encoding two isoforms of pokeweed antiviral protein in seeds (PAP-S1 and S2) and the N-glycosidase activities of their recombinant proteins on ribosomes and DNA in comparison with other isoforms. J. Biochem. 2002, 131, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.R.; Lord, J.M. Genetics of ribosome-inactivating proteins. Mini Rev. Med. Chem. 2004, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Citores, L.; Buonamici, L.; Ferreras, J.M.; de Benito, F.M.; Stirpe, F.; Girbes, T. Toxicity and cytotoxicity of nigrin b, a two-chain ribosome-inactivating protein from Sambucus nigra: Comparison with ricin. Arch. Toxicol. 1997, 71, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Duggar, B.M.; Armstrong, J.K. The effect of treating virus of tobacco mosaic with juice of various plants. Ann. Mol. Bot. Gard. 1925, 12, 360–364. [Google Scholar] [CrossRef]
- Dallal, J.A.; Irvin, J.D. Enzymatic inactivation of eukaryotic ribosomes by the pokeweed antiviral protein. FEBS Lett. 1978, 89, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, J.; Habuka, N.; Matuta, C.; Miyano, M.; Koiwai, A. Isolation and analysis of a genomic clone encoding a pokeweed antiviral protein. Plant Mol. Biol. 1992, 20, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Antoniw, J.F.; Hefferon, K.L.; Ivanov, I.G.; Abouhaidar, M.G. Expression of pokeweed (Phytolacca americana) antiviral protein cDNA in Escherichia coli and its antiviral activity. Physiol. Mol. Plant Path. 1993, 42, 237–247. [Google Scholar] [CrossRef]
- Xu, J.; Meng, A.X.; Hefferon, K.L.; Ivanov, I.G.; Abouhaidar, M.G. Effect of N-terminal deletions on the activity of pokeweed antiviral protein expressed in E. coli. Biochimie 1998, 80, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Kataoka, J.; Tsuge, H.; Habuka, N.; Inagaki, E.; Noma, M.; Miyano, M. X-ray structure of pokeweed antiviral protein, coded by a new gwnomic clone, at 0.23 nm resolution. A model structure provides a suitable electrostatic field for substrate binding. Eur. J. Biochem. 1994, 225, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Kung, S.S.; Kimura, Y.; Funatsu, G. N-acetyl-D-glucosamine-asparagine structure in ribosome-inactivating proteins from seeds of Luffa cylindrica and Phytolacca americana. Agric. Biol. Chem. 1991, 55, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Kurinov, I.V.; Uckun, F.M. High resolution X-ray structure of potent anti-HIV pokeweed antiviral protein-III. Biochem. Pharmacol. 2003, 65, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Kurinov, I.V.; Rajamohan, F.; Venkatachalam, T.K.; Uckun, F.M. X-ray crystallographic analysis of the structural basis for the interaction of pokeweed antiviral protein with guanine residues of ribosomal RNA. Protein Sci. 1999, 8, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, F.; Venkatachalam, T.K.; Irvin, J.D.; Uckun, F.M. Pokeweed antiviral protein isoforms PAP-I, PAP-II, and PAP-III depurinate RNA of human immunodeficiency virus (HIV)-1. Biochem. Biophys. Res. Commun. 1999, 260, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Barbieri, L.; Abbondanza, A.; Falasca, A.I.; Carnicelli, D.; Battelli, M.G.; Stirpe, F. Purification and properties of new ribosome-inactivating proteins with RNA N-glycosidase activity. Biochim. Biophys. Acta 1990, 1087, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lawrence, C.B.; Linden, J.C.; Vivanco, J.M. Isolation and characterization of a novel ribosome-inactivating protein from root cultures of pokeweed and its mechanism of secretion from roots. Plant Physiol. 2002, 130, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Bolognesi, A.; Cenini, P.; Falasca, A.I.; Minghetti, A.; Garofano, L.; Guicciardi, A.; Lappi, D.; Miller, S.P.; Stirpe, F. Ribosome-inactivating proteins from plant cells in culture. Biochem. J. 1989, 257, 801–807. [Google Scholar] [PubMed]
- Sperti, S.; Montanaro, L.; Mattioli, A.; Testoni, G. Relationship between elongation factor I- and elongation factor II- dependent guanosine triphosphatase activities of ribosomes. Inhibition of both activities by ricin. Biochem. J. 1975, 148, 447–451. [Google Scholar] [PubMed]
- Barbieri, L.; Ferreras, J.M.; Barraco, A.; Ricci, P.; Stirpe, F. Some ribosome-inactivating proteins depurinate ribosomal RNA at multiple sites. Biochem. J. 1992, 286, 1–4. [Google Scholar] [PubMed]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide: Adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [PubMed]
- Gutell, R.R.; Fox, G.E. A compilation of large subunit RNA sequences presented in a structural format. Nucleic Acids Res. 1988, 16, r175–r269. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Link, T.M.; Schramm, V.L. Ricin A-chain: kinetics, mechanism, and RNA stem-loop inhibitors. Biochemistry 1998, 37, 11605–11613. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.S.; Chen, X.Y.; Ichikawa, Y.; Tyler, P.C.; Furneaux, R.H.; Schramm, V.L. Ricin A-chain inhibitors resembling the oxacarbenium ion transition state. Biochemistry 2001, 40, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Nourollahzadeh, E.; Hudak, K.A. Pokeweed antiviral protein depurinates the sarcin/ricin loop of the rRNA prior to binding of aminoacyl-tRNA to the ribosomal A-site. RNA 2006, 12, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Hammell, A.B.; Yasenchak, J.; Tumer, N.E.; Dinman, J.D. A C-terminal deletion mutant of pokeweed antiviral protein inhibits programmed+1 ribosomal frameshifting and Ty1 retrotransposition without depurinating the sarcin/ricin loop of rRNA. Virology 2001, 279, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Tumer, N.E.; Parikh, B.A.; Li, P.; Dinman, J.D. The pokeweed antiviral protein specifically inhibits Ty1-directed +1 ribosomal frameshifting and retrotransposition in Saccharomyces cerevisiae. J. Virol. 1998, 72, 1036–1042. [Google Scholar] [PubMed]
- Vater, C.A.; Bartle, L.M.; Leszyk, J.D.; Lambert, J.M.; Goldmacher, V.S. Ricin A chain can be chemically cross-linked to the mammalian ribosomal proteins L9 and L10e. J. Biol. Chem. 1995, 270, 12933–12940. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Dinman, J.D.; Tumer, N.E. Pokeweed antiviral protein accesses ribosomes by binding to L3. J. Biol. Chem. 1999, 274, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Tumer, N.E. Expression of a truncated form of ribosomal protein L3 confers resistance to pokeweed antiviral protein and the Fusarium mycotoxin deoxynivalenol. Mol. Plant-Microbe Interact. 2005, 18, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Gluck, A.; Wool, I.G. Ribosomal RNA identity elements for ricin A-chain recognition and catalysis. J. Mol. Biol. 1991, 221, 193–207. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Dong, D.X.; Yin, X.W.; Sun, J.; Ren, H.J.; Li, R.X. Comparative analysis of depurination catalyzed by ricin A-chain on synthetic 32mer and 25mer oligoribonucleotides mimicking the sarcin/ricin domain of the rat 28S rRNA and E. coli 23S rRNA. J. Biotechnol. 2009, 139, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Domashevskiy, A.V.; Miyoshi, H.; Goss, D.J. Inhibition of pokeweed antiviral protein (PAP) by turnip mosaic virus genome-linked protein (VPg). J. Biol. Chem. 2012, 287, 29729–29738. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xie, L.; Hou, F.; Liu, W.Y.; Ruan, K. Non-specific deadenylation and deguanylation of naked RNA catalyzed by ricin under acidic condition. Biochim. Biophys. Acta 2001, 1519, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Brigotti, M.; Rambelli, F.; Montanaro, L.; Sperti, S. High-pressure-liquid-chromatographic and fluorimetric methods for the determination of adenine released from ribosomes by ricin and gelonin. Biochem. J. 1989, 259, 639–643. [Google Scholar] [PubMed]
- Rajamohan, F.; Kurinov, I.V.; Venkatachalam, T.K.; Uckun, F.M. Deguanylation of human immunodeficiency virus (HIV-1) RNA by recombinant pokeweed antiviral protein. Biochem. Biophys. Res. Commun. 1999, 263, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Rajamohan, F.; Pendergrass, S.; Ozer, Z.; Waurzyniak, B.; Mao, C. Structure-based design and engineering of a nontoxic recombinant pokeweed antiviral protein with potent anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 2003, 47, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Antoniw, J.F.; White, R.F. A possible mechanism for the antiviral activity of pokeweed antiviral protein. Physiol. Mol. Plant Path. 1993, 42, 249–258. [Google Scholar] [CrossRef]
- Ussery, M.A.; Irvin, J.D.; Hardesty, B. Inhibition of poliovirus replication by a plant antiviral peptide. Ann. NY Acad. Sci. 1977, 284, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Aron, G.M.; Irvin, J.D. Inhibition of herpes simplex virus multiplication by the pokeweed antiviral protein. Antimicrob. Agents Chemother. 1980, 17, 1032–1033. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.A.; Walker, V.M.; Flewett, T.H.; Barclay, G.R. The inhibition of infection by cucumber mosaic virus and influenza virus by extracts from Phytolacca americana. J. Gen. Virol. 1974, 22, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Kao, C.C.; Hudak, K.A. Pokeweed antiviral protein inhibits brome mosaic virus replication in plant cells. J. Biol. Chem. 2005, 280, 20069–20075. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Rustamova, L.; Vassilev, A.O.; Tibbles, H.E.; Petkevich, A.S. CNS activity of Pokeweed anti-viral protein (PAP) in mice infected with lymphocytic choriomeningitis virus (LCMV). BMC Infect. Dis. 2005, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishag, H.Z.; Li, C.; Huang, L.; Sun, M.X.; Ni, B.; Guo, C.X.; Mao, X. Inhibition of Japanese encephalitis virus infection in vitro and in vivo by pokeweed antiviral protein. Virus Res. 2013, 171, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Wang, P.; Tumer, N.E. A novel mechanism for inhibition of translation by pokeweed antiviral protein: Depurination of the capped RNA template. RNA 2000, 6, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Bauman, J.D.; Tumer, N.E. Pokeweed antiviral protein binds to the cap structure of eukaryotic mRNA and depurinates the mRNA downstream of the cap. RNA 2002, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.E.; Khan, M.A.; Tumer, N.E.; Goss, D.J.; Friedland, D.E. Characterization of pokeweed antiviral protein binding to mRNA cap analogs: Competition with nucleotides and enhancement by translation initiation factor iso4G. Biochim. Biophys. Acta 2009, 1789, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zoubenko, O.; Hudak, K.; Tumer, N.E. A non-toxic pokeweed antiviral protein mutant inhibits pathogen infection via a novel salicylic acid-independent pathway. Plant Mol. Biol. 2000, 44, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, J.M.; Tumer, N.E. Translation Inhibition of Capped and Uncapped Viral RNAs Mediated by Ribosome-Inactivating Proteins. Phytopathology 2003, 93, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hudak, K.A. A novel interaction of pokeweed antiviral protein with translation initiation factors 4G and iso4G: A potential indirect mechanism to access viral RNAs. Nucleic Acids Res. 2006, 34, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Metz, A.M.; Timmer, R.T.; Rhoads, R.E.; Browning, K.S. Isolation and sequence of the cDNAs encoding the subunits of the isozyme form of wheat protein synthesis initiation factor 4F. J. Biol. Chem. 1992, 267, 23232–23236. [Google Scholar] [PubMed]
- Browning, K.S. The plant translational apparatus. Plant Mol. Biol. 1996, 32, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Domashevskiy, A.; Kobilinsky, L. The Effect of Eukaryotic Initiation Factors on the Activity of Pokeweed Antiviral Protein; Society of Toxicology: Reston, VA, USA, 2014. [Google Scholar]
- Gallie, D.R.; Walbot, V. RNA pseudoknot domain of tobacco mosaic virus can functionally substitute for a poly(A) tail in plant and animal cells. Genes Dev. 1990, 4, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R.; Kobayashi, M. The role of the 3'-untranslated region of non-polyadenylated plant viral mRNAs in regulating translational efficiency. Gene 1994, 142, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Domashevskiy, A.V. Pokeweed antiviral protein binds to structures present in the 3' untranslated regions of viral mRNA. In Proceeding of Annual Biomedical Research Conference for Minority Students, San Antonio, TX, USA, 12–15 November 2014.
- Domashevskiy, A.V. The 3' Untranslated Regions within Viral RNA Affects Pokeweed Antiviral Protein Antiviral Activity; American Society of Biochemistry and Molecular Biology: Rockville, MD, USA, 2015. [Google Scholar]
- Sandvig, K.; van Deurs, B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000, 19, 5943–5950. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Peumans, W.J.; Barre, A.; Rouge, P. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Tumer, N.E.; Hudak, K.; Di, R.; Coetzer, C.; Wang, P.; Zoubenko, O. Pokeweed antiviral protein and its applications. Curr. Top Microbiol. Immunol. 1999, 240, 139–158. [Google Scholar] [PubMed]
- Dowd, P.F.; Zuo, W.N.; Gillikin, J.W.; Johnson, E.T.; Boston, R.S. Enhanced resistance to Helicoverpa zea in tobacco expressing an activated form of maize ribosome-inactivating protein. J. Agric. Food Chem. 2003, 51, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tumer, N.E. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 2000, 55, 325–355. [Google Scholar] [PubMed]
- Craig, H.L.; Alderks, O.H.; Corwin, A.H.; Dieke, S.H.; Karel, C.L. Preparation of toxic ricin. U.S. Patent 3,060,165, 1962. [Google Scholar]
- Smallshaw, J.E.; Firan, A.; Fulmer, J.R.; Ruback, S.L.; Ghetie, V.; Vitetta, E.S. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine 2002, 20, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S. UK doctors warned after ricin poison found in police raid. BMJ 2003, 326, 126. [Google Scholar] [CrossRef] [PubMed]
- Texas actress who sent Obama ricin sentenced to 18 years. Available online: http://www.cnn.com/2014/07/16/justice/texas-ricin-actress-sentenced/index.html (accessed on 27 January 2015).
- Tourlakis, M.E.; Karran, R.A.; Desouza, L.; Siu, K.W.; Hudak, K.A. Homodimerization of pokeweed antiviral protein as a mechanism to limit depurination of pokeweed ribosomes. Mol. Plant Path. 2010, 11, 757–767. [Google Scholar]
- Bolognesi, A.; Polito, L. Immunotoxins and other conjugates: Pre-clinical studies. Mini Rev. Med. Chem. 2004, 4, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Hudak, K.A.; Parikh, B.A.; Di, R.; Baricevic, M.; Santana, M.; Seskar, M.; Tumer, N.E. Generation of pokeweed antiviral protein mutations in Saccharomyces cerevisiae: Evidence that ribosome depurination is not sufficient for cytotoxicity. Nucleic Acids Res. 2004, 32, 4244–4256. [Google Scholar] [CrossRef] [PubMed]
- Lappi, D.A.; Wiley, R.G. Immunotoxins and neuropeptide-toxin conjugates experimental applications. Mini Rev. Med. Chem. 2004, 4, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.; Kersey, J.H.; Jaszcz, W.B.; Gunther, R.; Nguyen, D.P.; Chelstrom, L.M.; Tuel-Ahlgren, L.; Uckun, F.M. Effective immunochemotherapy of human t(4;11) leukemia in mice with severe combined immunodeficiency (SCID) using B43 (anti-CD19)-pokeweed antiviral protein immunotoxin plus cyclophosphamide. Leukemia 1993, 7, 290–297. [Google Scholar] [PubMed]
- Uckun, F.M.; Haissig, S.; Ledbetter, J.A.; Fidler, P.; Myers, D.E.; Kuebelbeck, V.; Weisdorf, D.; Gajl-Peczalska, K.; Kersey, J.H.; Ramsay, N.K. Developmental hierarchy during early human B-cell ontogeny after autologous bone marrow transplantation using autografts depleted of CD19+ B-cell precursors by an anti-CD19 pan-B-cell immunotoxin containing pokeweed antiviral protein. Blood 1992, 79, 3369–3379. [Google Scholar] [PubMed]
- Uckun, F.M.; Chelstrom, L.M.; Finnegan, D.; Tuel-Ahlgren, L.; Manivel, C.; Irvin, J.D.; Myers, D.E.; Gunther, R. Effective immunochemotherapy of CALLA+C mu+ human pre-B acute lymphoblastic leukemia in mice with severe combined immunodeficiency using B43 (anti-CD19) pokeweed antiviral protein immunotoxin plus cyclophosphamide. Blood 1992, 79, 3116–3129. [Google Scholar] [PubMed]
- Uckun, F.M.; Chelstrom, L.M.; Irvin, J.D.; Finnegan, D.; Gunther, R.; Young, J.; Kuebelbeck, V.; Myers, D.E.; Houston, L.L. In vivo efficacy of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin against BCL-1 murine B-cell leukemia. Blood 1992, 79, 2649–2661. [Google Scholar] [PubMed]
- Uckun, F.M.; Manivel, C.; Arthur, D.; Chelstrom, L.M.; Finnegan, D.; Tuel-Ahlgren, L.; Irvin, J.D.; Myers, D.E.; Gunther, R. In vivo efficacy of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin against human pre-B cell acute lymphoblastic leukemia in mice with severe combined immunodeficiency. Blood 1992, 79, 2201–2214. [Google Scholar] [PubMed]
- Erice, A.; Balfour, H.H., Jr.; Myers, D.E.; Leske, V.L.; Sannerud, K.J.; Kuebelbeck, V.; Irvin, J.D.; Uckun, F.M. Anti-human immunodeficiency virus type 1 activity of an anti-CD4 immunoconjugate containing pokeweed antiviral protein. Antimicrob. Agents Chemother. 1993, 37, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.M.; Moran, P.A.; Haffar, O.; Sias, J.; Richman, D.D.; Spina, C.A.; Myers, D.E.; Kuebelbeck, V.; Ledbetter, J.A.; Uckun, F.M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature 1990, 347, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Baluna, R.; Vitetta, E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ready, M.P.; Kim, Y.; Robertus, J.D. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins 1991, 10, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jasheway, K.; Pruet, J.; Anslyn, E.V.; Robertus, J.D. Structure-based design of ricin inhibitors. Toxins 2011, 3, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H.; Fauquet, C.M.; Bishop, D.H.; Carstens, E.B.; Esters, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Virus Taxonomy: Seventh Report of the Interanational Committee on Taxonomy of Viruses; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Murphy, J.F.; Rychlik, W.; Rhoads, R.E.; Hunt, A.G.; Shaw, J.G. A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J. Virol. 1991, 65, 511–513. [Google Scholar] [PubMed]
- Goodfellow, I.; Chaudhry, Y.; Gioldasi, I.; Gerondopoulos, A.; Natoni, A.; Labrie, L.; Laliberte, J.F.; Roberts, L. Calicivirus translation initiation requires an interaction between VPg and eIF 4 E. EMBO Rep. 2005, 6, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Plante, D.; Wittmann, S.; Daigneault, N.; Fortin, M.G.; Laliberte, J.F. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 2000, 74, 7730–7737. [Google Scholar] [CrossRef] [PubMed]
- Daughenbaugh, K.F.; Fraser, C.S.; Hershey, J.W.; Hardy, M.E. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003, 22, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Urcuqui-Inchima, S.; Haenni, A.L.; Bernardi, F. Potyvirus proteins: A wealth of functions. Virus Res. 2001, 74, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, S.; Chatel, H.; Fortin, M.G.; Laliberte, J.F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology 1997, 234, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Miyoshi, H.; Ray, S.; Natsuaki, T.; Suehiro, N.; Goss, D.J. Interaction of genome-linked protein (VPg) of turnip mosaic virus with wheat germ translation initiation factors eIFiso4E and eIFiso4F. J. Biol. Chem. 2006, 281, 28002–28010. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.F.; Klein, P.G.; Hunt, A.G.; Shaw, J.G. Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology 1996, 220, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Dunoyer, P.; Thomas, C.; Harrison, S.; Revers, F.; Maule, A. A cysteine-rich plant protein potentiates Potyvirus movement through an interaction with the virus genome-linked protein VPg. J. Virol. 2004, 78, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, O.; Dunnington, S.W.; Gotow, L.F.; Pirone, T.P.; Hellmann, G.M. Variations in the VPg protein allow a potyvirus to overcome va gene resistance in tobacco. Virology 1997, 237, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Rajamaki, M.L.; Valkonen, J.P. The 6K2 protein and the VPg of potato virus A are determinants of systemic infection in Nicandra physaloides. Mol. Plant-Microbe Interact. 1999, 12, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Rajamaki, M.L.; Valkonen, J.P. Viral genome-linked protein (VPg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves. Mol. Plant-Microbe Interact. 2002, 15, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Schaad, M.C.; Carrington, J.C. Suppression of long-distance movement of tobacco etch virus in a nonsusceptible host. J. Virol. 1996, 70, 2556–2561. [Google Scholar] [PubMed]
- Schaad, M.C.; Lellis, A.D.; Carrington, J.C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 1997, 71, 8624–8631. [Google Scholar] [PubMed]
- Rutenber, E.; Katzin, B.J.; Ernst, S.; Collins, E.J.; Mlsna, D.; Ready, M.P.; Robertus, J.D. Crystallographic refinement of ricin to 2.5 A. Proteins 1991, 10, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mlsna, D.; Monzingo, A.F.; Ready, M.P.; Frankel, A.; Robertus, J.D. Structure of a ricin mutant showing rescue of activity by a noncatalytic residue. Biochemistry 1992, 31, 3294–3296. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Miyoshi, H.; Gallie, D.R.; Goss, D.J. Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: Interactions with translation initiation factors eIF4F and eIFiso4F. J. Biol. Chem. 2008, 283, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Roudet-Tavert, G.; Michon, T.; Walter, J.; Delaunay, T.; Redondo, E.; Le Gall, O. Central domain of a potyvirus VPg is involved in the interaction with the host translation initiation factor eIF4E and the viral protein HcPro. J. Gen. Virol. 2007, 88, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Vitetta, E. Immunotoxins in the therapy of cancer: From bench to clinic. Pharmacol. Ther. 1994, 63, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Pai, L.H.; Pastan, I. Immunotoxin therapy for cancer. JAMA 1993, 269, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Tagge, E.P.; Willingham, M.C. Clinical trials of targeted toxins. Semin. Cancer Biol. 1995, 6, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, M.A.; Vitetta, E.S. Recent developments in immunotoxin therapy. Curr. Opin. Immunol. 1994, 6, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Grossbard, M.L.; Nadler, L.M. Immunotoxin therapy of lymphoid neoplasms. Semin. Hematol. 1994, 31, 88–97. [Google Scholar] [PubMed]
- Anderson, P.M.; Meyers, D.E.; Hasz, D.E.; Covalcuic, K.; Saltzman, D.; Khanna, C.; Uckun, F.M. In vitro and in vivo cytotoxicity of an anti-osteosarcoma immunotoxin containing pokeweed antiviral protein. Cancer Res. 1995, 55, 1321–1327. [Google Scholar] [PubMed]
- Waurzyniak, B.; Schneider, E.A.; Tumer, N.; Yanishevski, Y.; Gunther, R.; Chelstrom, L.M.; Wendorf, H.; Myers, D.E.; Irvin, J.D.; Messinger, Y.; et al. In vivo toxicity, pharmacokinetics, and antileukemic activity of TXU (anti-CD7)-pokeweed antiviral protein immunotoxin. Clin. Cancer Res. 1997, 3, 881–890. [Google Scholar] [PubMed]
- Allen, T.M.; Martin, F.J. Advantages of liposomal delivery systems for anthracyclines. Semin. Oncol. 2004, 31, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Medina, O.P.; Zhu, Y.; Kairemo, K. Targeted liposomal drug delivery in cancer. Curr. Pharm. Des. 2004, 10, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tuunainen, I.; Parry, M.; Medina, O.P.; Mancini, G.; Kinnunen, P.K. Binding of cationic liposomes to apoptotic cells. Anal. Biochem. 2004, 331, 385–394. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domashevskiy, A.V.; Goss, D.J. Pokeweed Antiviral Protein, a Ribosome Inactivating Protein: Activity, Inhibition and Prospects. Toxins 2015, 7, 274-298. https://doi.org/10.3390/toxins7020274
Domashevskiy AV, Goss DJ. Pokeweed Antiviral Protein, a Ribosome Inactivating Protein: Activity, Inhibition and Prospects. Toxins. 2015; 7(2):274-298. https://doi.org/10.3390/toxins7020274
Chicago/Turabian StyleDomashevskiy, Artem V., and Dixie J. Goss. 2015. "Pokeweed Antiviral Protein, a Ribosome Inactivating Protein: Activity, Inhibition and Prospects" Toxins 7, no. 2: 274-298. https://doi.org/10.3390/toxins7020274
APA StyleDomashevskiy, A. V., & Goss, D. J. (2015). Pokeweed Antiviral Protein, a Ribosome Inactivating Protein: Activity, Inhibition and Prospects. Toxins, 7(2), 274-298. https://doi.org/10.3390/toxins7020274