Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework
Abstract
:1. Introduction


2. Results and Discussion
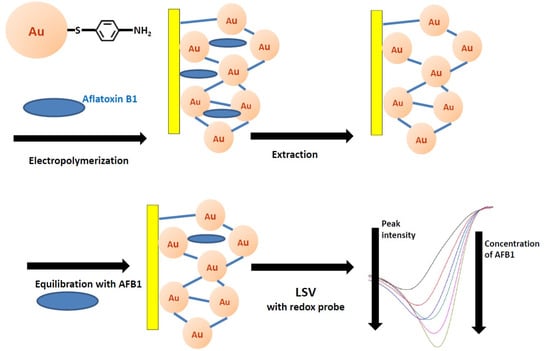
2.1. Fabrication of Aflatoxin B1 Imprinted MOF Film
2.1.1. Characterization of PATP Functionalized AuNPs


2.1.2. Electropolymerization of AFB1 MIP-MOF Film

2.1.3. Structural and Electrochemical Characterization of AFB1 MIP-MOF Film


2.2. Recognition Ability of MIP MOF Films toward Aflatoxin B1
2.2.1. Electrochemical Detection of AFB1 Recognition by MIP-MOF Film

2.2.2. Mechanism of AFB1 Recognition by MIP-MOF Film
2.3. Analytical Performance of the MIP MOF Sensor

| Method | LOD | Linear Range | Reference |
|---|---|---|---|
| Immunosensor on screen-printed carbon electrodes | 90 pg/mL | 0.1–10 ng/mL | [25] |
| Immunosensor on screen-printed microplate | 30 pg/mL | 0.05–2 ng/mL | [26] |
| Immunosensor on glassy carbon electrode | 0.07 ng/mL | 0.6–2.4 ng/mL | [27] |
| HPLC | 0.25 ng/mL | 0.5–10 ng/mL | [28] |
| HPLC-MSMS | 0.08 ng/mL | 0.3–10 ng/mL | [29] |
| LC-MSMS | 2.4 pg/mL | 0.05–2 ng/mL | [6] |
| LSV on molecularly imprinted MOF | 0.3 fg/mL | 1 fg/mL–1 μg/mL | Present method |

| Type of Mycotoxin | Cross-reactivity (%) |
|---|---|
| Aflatoxin B2 | 10−7 |
| Aflatoxin G1 | 10−7 |
| Ochratoxin A | 10−5 |
3. Experimental Section
3.1. Chemicals and Instrumentation
3.2. Preparation of PATP Functionalized AuNPs
3.3. Preparation of MIP and Non-Imprinted (NIP) MOF Modified Electrodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Šegvić Klarić, M.; Rašić, D.; Peraica, M. Deleterious Effects of Mycotoxin Combinations Involving Ochratoxin A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives and Contaminants, WHO Food Additives Series 59; WHO: Geneva, Switzerland, 2008; pp. 305–356. [Google Scholar]
- Balzer, I.; Bogdanic, C.; Pepeljnjak, S. Rapid thin layer chromatographic method for determining aflatoxin B1, ochratoxin A, and zearalenone in corn. J. Assoc. Off. Anal. Chem. 1978, 61, 584–585. [Google Scholar] [PubMed]
- Hu, Y.Y.; Zheng, P.; Zhang, Z.X.; He, Y.Z. Determination of aflatoxins in high-pigment contents samples by matrix solid-phase dispersion and high-performance liquid chromatography. Asia-Australas. J. Anim. Sci. 2015, 28, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Gu, X.; Li, J.G.; Yao, T.; Dong, Y.C. Quality Evaluation of Five Commercial Enzyme Linked Immunosorbent Assay Kits for Detecting Aflatoxin B1 in Feedstuffs. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar]
- Nonaka, Y.; Saito, K.; Hanioka, N.; Narimatsu, S.; Kataoka, H. Determination of aflatoxins in food samples by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Diltemiz, S.E.; Hur, D.; Ersoz, A.; Denizli, A.; Say, R. Designing of MIP based QCM sensor having thymine recognition sites based on biomimicking DNA approach. Biosens. Bioelectron. 2009, 25, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Akhoundian, M. A novel potentiometric sensor for promethazine based on a molecularly imprinted polymer (MIP): The role of MIP structure on the sensor performance. Electrochim. Acta 2010, 55, 3477–3485. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wei, X.; Tao, H.; Pan, H. Molecularly Imprinted Electrochemical Luminescence Sensor Based On Signal Amplification for Selective Determination of Trace Gibberellin A3. Anal. Chem. 2012, 84, 9951–9955. [Google Scholar] [CrossRef] [PubMed]
- Suriyanarayanan, S.; Cywinski, P.J.; Moro, A.J.; Mohr, G.J.; Kutner, W. Chemosensors Based on Molecularly Imprinted Polymers. In Molecular Imprinting; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 325, pp. 165–265. [Google Scholar]
- Schirhagl, R. Bioapplications for Molecularly Imprinted Polymers. Anal. Chem. 2013, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Karube, I. Molecularly imprinted polymers for biosensor applications. Trac. Trends Anal. Chem. 1999, 18, 199–204. [Google Scholar] [CrossRef]
- Tokonami, S.; Shiigi, H.; Nagaoka, T. Review: micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal. Chim. Acta 2009, 641, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bondi, M.C.; Benito-Pena, M.E.; Urraca, J.L.; Orellana, G. Immuno-Like Assays and Biomimetic Microchips. In Molecular Imprinting; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 325, pp. 111–164. [Google Scholar]
- Apodaca, D.C.; Pernites, R.B.; Ponnapati, R.R.; Del Mundo, F.R.; Advincula, R.C. Electropolymerized molecularly imprinted polymer films of a bis-terthiophene dendron: folic acid quartz crystal microbalance sensing. ACS Appl. Mater. Interfaces 2011, 3, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.L. Hydrothermal synthesis of a metal organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Pan, L.; Parker, B.; Huang, X.; Olson, D.H.; Lee, J.; Li, J. Zn(tbip) (H2tbip = 5-tert-Butyl Isophthalic Acid): A Highly Stable Guest-Free Microporous Metal Organic Framework with Unique Gas Separation Capability. J. Am. Chem. Soc. 2006, 128, 4180–4181. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Ma, T.Y.; Li, H.; Deng, Q.F.; Liu, L.; Ren, T.Z.; Yuan, Z.Y. Ordered Mesoporous Metal-Organic Frameworks Consisting of Metal Disulfonates. Chem. Mater. 2012, 24, 2253–2255. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New Microporous Metal-Organic Framework Demonstrating Unique Selectivity for Detection of High Explosives and Aromatic Compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Florea, A.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Zhang, A.; Săndulescu, R.; Lagarde, F.; Jaffrezic-Renault, N. 1,3,5-Trinitrotoluene detection by a molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework. Sens. Actuators B 2015, 207, 960–966. [Google Scholar] [CrossRef]
- Florea, A.; Guo, Z.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Dzyadevych, S.; Săndulescu, R.; Jaffrezic-Renault, N. Anticancer drug detection using a highly sensitive molecularly imprinted electrochemical sensor based on an electropolymerized microporous metal organic framework. Talanta 2015, 138, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.T.; Navale, S.T.; Patil, V.B. Room temperature NO2 gas sensing properties of DBSA doped PPy-WO3 hybrid nanocomposite sensor. Org. Electron. 2015, 19, 15–25. [Google Scholar] [CrossRef]
- Ammida, N.; Micheli, L.; Piermarini, S.; Moscone, D.; Palleschi, G. Detection of aflatoxin B1 in barley: Comparative study of immunosensor and HPLC. Anal. Lett. 2006, 39, 1559–1572. [Google Scholar] [CrossRef] [Green Version]
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owino, J.H.; Arotiba, O.A.; Hendricks, N.; Songa, E.A.; Jahed, N.; Waryo, T.T.; Ngece, R.F.; Baker, P.G.; Iwuoha, E.I. Electrochemical Immunosensor Based on Polythionine/Gold Nanoparticles for the Determination of Aflatoxin B1. Sensors 2008, 8, 8262–8274. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zheng, P.; Zhang, Z.X.; He, Y.Z. Determination of Aflatoxins in High-Pigment Content Samples by Matrix Solid-Phase Dispersion and High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 4126–4130. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zheng, Y.; Luan, L.; Cai, Z.; Ren, Y.; Wu, Y. An ultra-high-performance liquid chromatography tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal. Chim. Acta 2010, 664, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Debouttière, P.J.; Roux, S.; Vocanson, F.; Billotey, C.; Beuf, O.; Favre-Réguillon, A.; Lin, Y.; Pellet-Rostaing, S.; Lamartine, R.; Perriat, P.; et al. Design of Gold Nanoparticles for Magnetic Resonance Imaging. Adv. Funct. Mater. 2006, 16, 2330–2339. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins 2015, 7, 3540-3553. https://doi.org/10.3390/toxins7093540
Jiang M, Braiek M, Florea A, Chrouda A, Farre C, Bonhomme A, Bessueille F, Vocanson F, Zhang A, Jaffrezic-Renault N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins. 2015; 7(9):3540-3553. https://doi.org/10.3390/toxins7093540
Chicago/Turabian StyleJiang, Mengjuan, Mohamed Braiek, Anca Florea, Amani Chrouda, Carole Farre, Anne Bonhomme, Francois Bessueille, Francis Vocanson, Aidong Zhang, and Nicole Jaffrezic-Renault. 2015. "Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework" Toxins 7, no. 9: 3540-3553. https://doi.org/10.3390/toxins7093540
APA StyleJiang, M., Braiek, M., Florea, A., Chrouda, A., Farre, C., Bonhomme, A., Bessueille, F., Vocanson, F., Zhang, A., & Jaffrezic-Renault, N. (2015). Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins, 7(9), 3540-3553. https://doi.org/10.3390/toxins7093540