Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor
Abstract
:1. Introduction
2. Results
2.1. Identification of Dermaseptin-PD-1 and Dermaseptin-PD-2 Precursor cDNAs from a Skin Secretion-Derived cDNA Library
2.2. Isolation and Structural Characterisation of Dermaseptin-PD-1 and Dermaseptin-PD-2
2.3. Solid Phase Synthesis of Dermaseptin-PD-1 and Dermaseptin-PD-2
2.4. Antimicrobial and Cytotoxic Activities
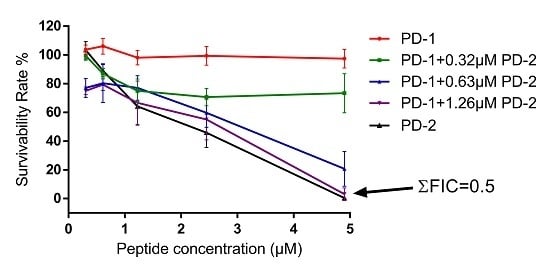
2.5. Assessment of Activity of Peptides on Human Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Acquisition and Maintenance of Experimental Specimens
4.2. Construction of a Skin Secretion-Derived cDNA Library and Cloning of Relevant cDNAs
4.3. Reverse Phase HPLC Fractionation of Crude Skin Secretion and Amino-Acid Sequence Analysis of Relevant Peptides
4.4. Solid-Phase Peptide Synthesis
4.5. Antimicrobial Assays
4.6. Haemolysis Assay
4.7. Assessment of Effects of Peptides on Human Cancer Cell Lines Using the MTT Assay
4.8. Assessment of Possible Synergism between the Two Peptides
Supplementary Materials
Data Deposition Footnote
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| HPLC | High performance liquid chromatography |
| cDNA | complmentary deoxyribonucleic acid |
| BLAST | Basic Local Alignment Search Tool |
| NCBI | National Centre for Biotechnological Information |
| MALDI-TOF | Matrix-assisted laser desorption/ionisation, time-of-flight |
| MS | mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| SEM | Standard error of the mean |
| TFA | Trifluoroacetic acid |
| PS | Peptide synthesiser |
| LC/MS/MS | Liquid chromatography coupled tandem mass spectrometry |
References
- Brown, K.L.; Hancock, R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Pept. Sci. 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Amiche, M.; Seon, A.A.; Wroblewski, H.; Nicolas, P. Isolation of dermatoxin from frog skin, an antibacterial peptide encoded by a novel member of the dermaseptin genes family. Eur. J. Biochem. 2000, 267, 4583–4592. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Nicolas, P. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 1994, 219, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Vanhoye, D.; Bruston, F.; Nicolas, P.; Amiche, M. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 2003, 270, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F.; Vanhoye, D.; Nicolas, P. Roles of diversifying selection and coordinated evolution in the evolution of amphibian antimicrobial peptides. Mol. Biol. Evol. 2002, 19, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.G.; Poulter, L.; Gibson, B.W.; Williams, D.H. Biosynthesis and degradation of peptides derived from xenopus laevis prohormones. Biochem. J. 1987, 243, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a class of antimicrobial peptides from xenopus skin: Isolation, characterization of two active forms, and partial cdna sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.S.; Poulter, L.; Williams, D.H.; Nutkins, J.C.; Giovannini, M.; Moore, C.; Gibson, B. The cDNA sequence coding for prepro-PGS (prepro-magainins) and aspects of the processing of this prepro-polypeptide. J. Biol. Chem. 1988, 263, 5745–5751. [Google Scholar] [PubMed]
- Fox, J. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.W.; Tang, D.; Mandrell, R.; Kelly, M.; Spindel, E. Bombinin-like peptides with antimicrobial activity from skin secretions of the asian toad, bombina orientalis. J. Biol. Chem. 1991, 266, 23103–23111. [Google Scholar] [PubMed]
- Simmaco, M.; Barra, D.; Chiarini, F.; Noviello, L.; Melchiorri, P.; Kreil, G.; Richter, K. A family of bombinin-related peptides from the skin of bombina variegata. Eur. J. Biochem. 1991, 199, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Barra, D.; Bossa, F. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J. Biol. Chem. 1994, 269, 11956–11961. [Google Scholar] [PubMed]
- Morikawa, N.; Hagiwara, K.I.; Nakajima, T. Brevinin-1 and-2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Ghavami, S.; Asoodeh, A.; Klonisch, T.; Halayko, A.; Kadkhoda, K.; Kroczak, T.; Gibson, S.; Booy, E.; Naderi-Manesh, H.; Los, M. Brevinin-2r(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J. Cell. Mol. Med. 2008, 12, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Van Huong, N.; Delfour, A.; Migliore-Samour, D.; Nicolas, P. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 1991, 30, 8824–8830. [Google Scholar] [CrossRef] [PubMed]
- Belaid, A.; Hani, K. Antiviral and antifungal activity of some dermaseptin s4 analogues. Afr. J. Biotechnol. 2011, 10, 14962–14967. [Google Scholar] [CrossRef]
- Bergaoui, I.; Zairi, A.; Tangy, F.; Aouni, M.; Selmi, B.; Hani, K. In vitro antiviral activity of dermaseptin s4 and derivatives from amphibian skin against herpes simplex virus type 2. J. Med. Virol. 2013, 85, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Hani, K.; Nicolas, P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J. Biol. Chem. 1994, 269, 31635–31641. [Google Scholar] [PubMed]
- Hernandez, C.; Mor, A.; Dagger, F.; Nicolas, P.; Hernandez, A.; Benedetti, E.; Dunia, I. Functional and structural damage in leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 1992, 59, 414. [Google Scholar] [PubMed]
- Brand, G.D.; Leite, J.R.S.; de Sá Mandel, S.M.; Mesquita, D.A.; Silva, L.P.; Prates, M.V.; Barbosa, E.A.; Vinecky, F.; Martins, G.R.; Galasso, J.H. Novel dermaseptins from Phyllomedusa hypochondrialis (Amphibia). Biochem. Biophys. Res. Commun. 2006, 347, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Brand, G.D.; Leite, J.R.S.; Silva, L.P.; Albuquerque, S.; Prates, M.V.; Azevedo, R.B.; Carregaro, V.; Silva, J.S.; Sá, V.C.; Brandão, R.A. Dermaseptins from Phyllomedusa oreades andphyllomedusa distincta anti-trypanosoma cruzi activity without cytotoxicity to mammalian cells. J. Biol. Chem. 2002, 277, 49332–49340. [Google Scholar] [CrossRef] [PubMed]
- Amiche, M.; Ladram, A.; Nicolas, P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily phyllomedusinae. Peptides 2008, 29, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Strahilevitz, J.; Mor, A.; Nicolas, P.; Shai, Y. Spectrum of antimicrobial activity and assembly of dermaseptin-b and its precursor form in phospholipid membranes. Biochemistry 1994, 33, 10951–10960. [Google Scholar] [CrossRef] [PubMed]
- Van Zoggel, H.; Hamma-Kourbali, Y.; Galanth, C.; Ladram, A.; Nicolas, P.; Courty, J.; Amiche, M.; Delbe, J. Antitumor and angiostatic peptides from frog skin secretions. Amino Acids 2012, 42, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-tasser: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Wechselberger, C. Cloning of cDNAs encoding new peptides of the dermaseptin-family. Biochim. Biophys. Acta 1998, 1388, 279–283. [Google Scholar] [CrossRef]
- Shai, Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 1995, 20, 460–464. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Hou, X.; Wang, L.; Ge, L.; Luo, Y.; Ma, C.; Zhou, M.; Duan, J.; Chen, T.; Shaw, C. Feleucins: Novel bombinin precursor-encoded nonapeptide amides from the skin secretion of Bombina variegata. BioMed Res. Int. 2014, 2014, 617362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Middleton, R.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983, 11, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, Y.; Liu, D.; Chen, Y. Two hits are better than one: Synergistic anticancer activity of α-helical peptides and doxorubicin/epirubicin. Oncotarget 2015, 6, 1769–1778. [Google Scholar] [CrossRef] [PubMed]









| #1 | b(1+) | b(2+) | Sequence | y(1+) | y(2+) | #2 |
|---|---|---|---|---|---|---|
| 1 | 58.0288 | 29.518 | G | - | - | 31 |
| 2 | 189.0693 | 95.0383 | M | 3207.7053 | 1604.3563 | 30 |
| 3 | 375.1486 | 188.0779 | W | 3076.6648 | 1538.836 | 29 |
| 4 | 462.1806 | 231.5939 | S | 2890.5855 | 1445.7964 | 28 |
| 5 | 590.2756 | 295.6414 | K | 2803.5534 | 1402.2804 | 27 |
| 6 | 703.3596 | 352.1835 | I | 2675.4584 | 1338.2329 | 26 |
| 7 | 831.4546 | 416.2309 | K | 2562.3744 | 1281.6908 | 25 |
| 8 | 960.4972 | 480.7522 | E | 2434.2794 | 1217.6433 | 24 |
| 9 | 1061.5449 | 531.2761 | T | 2305.2368 | 1153.122 | 23 |
| 10 | 1132.582 | 566.7946 | A | 2204.1891 | 1102.5982 | 22 |
| 11 | 1263.6225 | 632.3149 | M | 2133.152 | 1067.0796 | 21 |
| 12 | 1334.6596 | 667.8335 | A | 2002.1115 | 1001.5594 | 20 |
| 13 | 1405.6968 | 703.352 | A | 1931.0744 | 966.0408 | 19 |
| 14 | 1476.7339 | 738.8706 | A | 1860.0373 | 930.5223 | 18 |
| 15 | 1604.8288 | 802.9181 | K | 1789.0002 | 895.0037 | 17 |
| 16 | 1733.8714 | 867.4394 | E | 1660.9052 | 830.9562 | 16 |
| 17 | 1804.9086 | 902.9579 | A | 1531.8626 | 766.4349 | 15 |
| 18 | 1875.9457 | 938.4765 | A | 1460.8255 | 730.9164 | 14 |
| 19 | 2004.0407 | 1002.524 | K | 1389.7883 | 695.3978 | 13 |
| 20 | 2075.0778 | 1038.0425 | A | 1261.6934 | 631.3503 | 12 |
| 21 | 2146.1149 | 1073.5611 | A | 1190.6563 | 595.8318 | 11 |
| 22 | 2203.1364 | 1102.0718 | G | 1119.6191 | 560.3132 | 10 |
| 23 | 2331.2313 | 1166.1193 | K | 1062.5977 | 531.8025 | 9 |
| 24 | 2432.279 | 1216.6431 | T | 934.5027 | 467.755 | 8 |
| 25 | 2545.3631 | 1273.1852 | I | 833.455 | 417.2311 | 7 |
| 26 | 2632.3951 | 1316.7012 | S | 720.3709 | 360.6891 | 6 |
| 27 | 2747.4221 | 1374.2147 | D | 633.3389 | 317.1731 | 5 |
| 28 | 2878.4626 | 1439.7349 | M | 518.312 | 259.6596 | 4 |
| 29 | 2991.5466 | 1496.277 | I | 387.2715 | 194.1394 | 3 |
| 30 | 3119.6416 | 1560.3244 | K | 274.1874 | 137.5973 | 2 |
| 31 | - | - | Q-Amidated | 146.0924 | 73.5499 | 1 |
| #1 | b(1+) | b(2+) | Sequence | y(1+) | y(2+) | #2 |
|---|---|---|---|---|---|---|
| 1 | 58.0288 | 29.518 | G | - | - | 33 |
| 2 | 189.0693 | 95.0383 | M | 3111.7462 | 1556.3767 | 32 |
| 3 | 375.1486 | 188.0779 | W | 2980.7057 | 1490.8565 | 31 |
| 4 | 462.1806 | 231.5939 | S | 2794.6263 | 1397.8168 | 30 |
| 5 | 590.2756 | 295.6414 | K | 2707.5943 | 1354.3088 | 29 |
| 6 | 703.3596 | 352.1835 | I | 2579.4993 | 1290.2533 | 28 |
| 7 | 831.4546 | 416.2309 | K | 2466.4153 | 1233.7113 | 27 |
| 8 | 945.4975 | 473.2524 | N | 2338.3203 | 1169.6638 | 26 |
| 9 | 1016.5347 | 508.771 | A | 2224.2774 | 1112.6423 | 25 |
| 10 | 1073.5561 | 537.2817 | G | 2153.2402 | 1077.1238 | 24 |
| 11 | 1201.6511 | 601.3292 | K | 2096.2188 | 1048.613 | 23 |
| 12 | 1272.6882 | 636.8478 | A | 1968.1238 | 984.5655 | 22 |
| 13 | 1343.7253 | 672.3663 | A | 1897.0867 | 949.047 | 21 |
| 14 | 1414.7625 | 707.8849 | A | 1826.0496 | 913.5284 | 20 |
| 15 | 1542.8574 | 771.9324 | K | 1755.0124 | 878.0099 | 19 |
| 16 | 1613.8946 | 807.4509 | A | 1626.9175 | 813.9624 | 18 |
| 17 | 1684.9317 | 842.9695 | A | 1555.8804 | 778.4438 | 17 |
| 18 | 1755.9688 | 878.488 | A | 1484.8432 | 742.9253 | 16 |
| 19 | 1884.0638 | 942.5355 | K | 1413.8061 | 707.4067 | 15 |
| 20 | 1955.1009 | 978.0541 | A | 1285.7111 | 643.3592 | 14 |
| 21 | 2026.138 | 1013.5726 | A | 1214.674 | 607.8407 | 13 |
| 22 | 2083.1595 | 1042.0834 | G | 1143.6369 | 572.3221 | 12 |
| 23 | 2211.2544 | 1106.1309 | K | 1086.6154 | 543.8114 | 11 |
| 24 | 2282.2916 | 1141.6494 | A | 958.5205 | 479.7639 | 10 |
| 25 | 2353.3287 | 1177.168 | A | 887.4833 | 444.2453 | 9 |
| 26 | 2466.4128 | 1233.71 | L | 816.4462 | 408.7268 | 8 |
| 27 | 2581.4397 | 1291.2235 | D | 703.3622 | 352.1847 | 7 |
| 28 | 2652.4768 | 1326.7421 | A | 588.3352 | 294.6712 | 6 |
| 29 | 2751.5452 | 1376.2763 | V | 517.2981 | 259.1527 | 5 |
| 30 | 2838.5773 | 1419.7923 | S | 418.2297 | 209.6185 | 4 |
| 31 | 2967.6199 | 1484.3136 | E | 331.1976 | 166.1025 | 3 |
| 32 | 3038.657 | 1519.8321 | A | 202.155 | 101.5812 | 2 |
| 33 | - | - | I-Amidated | 131.1179 | 66.0626 | 1 |
| Peptides | MIC (μM) | MBC (μM) | Hemolysis (μM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | C. albicans | P. aeruginosa | E. coli | S. aureus | C. albicans | P. aeruginosa | Horse Red Cells | |
| Dermaseptin-PD-1 | 19.6 | 39.2 | 39.2 | 19.6 | 39.2 | 78.4 | 78.4 | 78.4 | >156.8 |
| Dermaseptin-PD-2 | 5.0 | 5.0 | 10.1 | 2.5 | 20.2 | 10.1 | 20.2 | 10.1 | >161.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Hou, X.; Wang, L.; Gao, Y.; Wu, D.; Xi, X.; Zhou, M.; Kwok, H.F.; Duan, J.; Chen, T.; et al. Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins 2016, 8, 144. https://doi.org/10.3390/toxins8050144
Shi D, Hou X, Wang L, Gao Y, Wu D, Xi X, Zhou M, Kwok HF, Duan J, Chen T, et al. Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins. 2016; 8(5):144. https://doi.org/10.3390/toxins8050144
Chicago/Turabian StyleShi, Daning, Xiaojuan Hou, Lei Wang, Yitian Gao, Di Wu, Xinping Xi, Mei Zhou, Hang Fai Kwok, Jinao Duan, Tianbao Chen, and et al. 2016. "Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor" Toxins 8, no. 5: 144. https://doi.org/10.3390/toxins8050144
APA StyleShi, D., Hou, X., Wang, L., Gao, Y., Wu, D., Xi, X., Zhou, M., Kwok, H. F., Duan, J., Chen, T., & Shaw, C. (2016). Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins, 8(5), 144. https://doi.org/10.3390/toxins8050144





_Kwok.png)