Impact of CDT Toxin on Human Diseases
Abstract
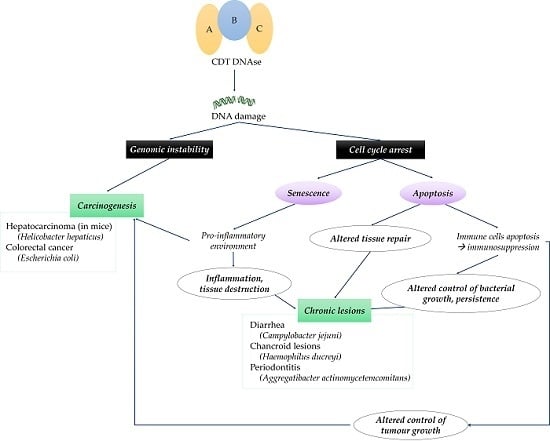
:1. Introduction
2. Role of CDT in Inflammation, Modulation of Immune Response and Tissue Damage
3. CDT-Producing Bacteria in Diseases
3.1. Camplylobacter jejuni Producing CDT and Diarrhea
3.2. Haemophilus ducreyi Producing CDT and Chancroid Lesions
3.3. Aggregatibacter (Actinobacillus) actinomycetemcomitans Producing CDT and Periodontitis
4. Potential Involvement of CDT in Cancers
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Elwell, C.A.; Dreyfus, L.A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 2000, 37, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galán, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins 2014, 6, 3098–3116. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Fedor, Y.; Vignard, J.; Nicolau-Travers, M.-L.; Boutet-Robinet, E.; Watrin, C.; Salles, B.; Mirey, G. From single-strand breaks to double-strand breaks during S-phase: A new mode of action of the Escherichia coli Cytolethal Distending Toxin. Cell. Microbiol. 2013, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bratti, X.; Karlsson, C.; Lagergård, T.; Thelestam, M.; Frisan, T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 2001, 276, 5296–5302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sharipo, A.; Chaves-Olarte, E.; Masucci, M.G.; Levitsky, V.; Thelestam, M.; Frisan, T. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cell. Microbiol. 2002, 4, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Fahrer, J.; Huelsenbeck, J.; Jaurich, H.; Dörsam, B.; Frisan, T.; Eich, M.; Roos, W.P.; Kaina, B.; Fritz, G. Cytolethal distending toxin (CDT) is a radiomimetic agent and induces persistent levels of DNA double-strand breaks in human fibroblasts. DNA Repair 2014, 18, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bratti, X.; Chaves-Olarte, E.; Lagergård, T.; Thelestam, M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J. Clin. Invest. 1999, 103, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Derheimer, F.A.; Kastan, M.B. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett. 2010, 584, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Schauer, D.B.; Fox, J.G. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell. Microbiol. 2008, 10, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Bayles, D.O. The Contribution of Cytolethal Distending Toxin to Bacterial Pathogenesis. Crit. Rev. Microbiol. 2006, 32, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Oswald, E.; Nougayrède, J.-P.; Taieb, F.; Sugai, M. Bacterial toxins that modulate host cell-cycle progression. Curr. Opin. Microbiol. 2005, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.A.; Balbo, P.B.; Pesci, E.C.; Cottle, D.L.; Mirabito, P.M.; Pickett, C.L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 1998, 66, 1934–1940. [Google Scholar] [PubMed]
- Svensson, L.A.; Henning, P.; Lagergård, T. The cytolethal distending toxin of Haemophilus ducreyi inhibits endothelial cell proliferation. Infect. Immun. 2002, 70, 2665–2669. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.; Johansson, A.; Wang, Y.; Claesson, R.; Chen, C.; Asikainen, S.; Kalfas, S. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. Eur. J. Oral Sci. 2002, 110, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Blazkova, H.; Krejcikova, K.; Moudry, P.; Frisan, T.; Hodny, Z.; Bartek, J. Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J. Cell. Mol. Med. 2010, 14, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Mattsson, A.; Wang, Y.; Chen, C.; Johansson, A. Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2004, 112, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Chao, K.; Patel, K.; Dreyfus, L. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 2001, 69, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Hassane, D.C.; Lee, R.B.; Pickett, C.L. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 2003, 71, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Majam, G.; Guerry, P. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect. Immun. 2005, 73, 5194–5197. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Hoffmaster, R.H.; Zekavat, A.; Yamaguchi, N.; Lally, E.T.; Demuth, D.R. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. (Baltim. Md. 1950) 2001, 167, 435–441. [Google Scholar] [CrossRef]
- Frisan, T.; Cortes-Bratti, X.; Chaves-Olarte, E.; Stenerlöw, B.; Thelestam, M. The Haemophilus ducreyi cytolethal distending toxin induces DNA double-strand breaks and promotes ATM-dependent activation of RhoA. Cell. Microbiol. 2003, 5, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Carr, H.S.; Richter-Dahlfors, A.; Masucci, M.G.; Thelestam, M.; Frost, J.A.; Frisan, T. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS ONE 2008, 3, e2254. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; McKay, T.; Datar, S.; Miller, M.; Chowhan, R.; Demuth, D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. (Baltim. Md. 1950) 1999, 162, 4773–4780. [Google Scholar]
- Shenker, B.J.; Dlakic, M.; Walker, L.P.; Besack, D.; Jaffe, E.; LaBelle, E.; Boesze-Battaglia, K. A novel mode of action for a microbial-derived immunotoxin: The cytolethal distending toxin subunit B exhibits phosphatidylinositol 3,4,5-triphosphate phosphatase activity. J. Immunol. (Baltim. Md. 1950) 2007, 178, 5099–5108. [Google Scholar] [CrossRef]
- Shenker, B.J.; Walker, L.P.; Zekavat, A.; Boesze-Battaglia, K. Lymphoid susceptibility to the Aggregatibacter actinomycetemcomitans cytolethal distending toxin is dependent upon baseline levels of the signaling lipid, phosphatidylinositol-3,4,5-triphosphate. Mol. Oral Microbiol. 2015, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Feng, Y.; Whary, M.T.; Nambiar, P.R.; Xu, S.; Ng, V.; Taylor, N.S.; Fox, J.G. Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred Swiss Webster mice. Infect. Immun. 2005, 73, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.S.; Sachen, K.L.; Wood, H.D.; Eaton, K.A.; Young, V.B. Modulation of host immune responses by the cytolethal distending toxin of Helicobacter hepaticus. Infect. Immun. 2006, 74, 4496–4504. [Google Scholar] [CrossRef] [PubMed]
- Rabin, S.D.P.; Flitton, J.G.; Demuth, D.R. Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Induces Apoptosis in Nonproliferating Macrophages by a Phosphatase-Independent Mechanism. Infect. Immun. 2009, 77, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Bezine, E.; Vignard, J.; Mirey, G. The Cytolethal Distending Toxin Effects on Mammalian Cells: A DNA Damage Perspective. Cells 2014, 3, 592–615. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; McVeigh, A.L.; Scott, D.A.; Michielutti, R.E.; Bixby, A.; Carroll, S.A.; Bourgeois, A.L.; Guerry, P. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 2000, 68, 6535–6541. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Walker, L.P.; Zekavat, A.; Dlakić, M.; Boesze-Battaglia, K. Blockade of the PI-3K signalling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines. Cell. Microbiol. 2014, 16, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Rogers, A.B.; Rickman, B.; Whary, M.T.; Xu, S.; Clapp, K.M.; Boutin, S.R.; Fox, J.G. Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infect. Immun. 2009, 77, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Abuoun, M.; Manning, G.; Cawthraw, S.A.; Ridley, A.; Ahmed, I.H.; Wassenaar, T.M.; Newell, D.G. Cytolethal distending toxin (CDT)-negative Campylobacter jejuni strains and anti-CDT neutralizing antibodies are induced during human infection but not during colonization in chickens. Infect. Immun. 2005, 73, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Ando, E.S.; De-Gennaro, L.A.; Faveri, M.; Feres, M.; DiRienzo, J.M.; Mayer, M.P.A. Immune response to cytolethal distending toxin of Aggregatibacter actinomycetemcomitans in periodontitis patients. J. Periodontal Res. 2010, 45, 471–480. [Google Scholar] [PubMed]
- Mbwana, J.; Ahmed, H.J.; Ahlman, K.; Sundaeus, V.; Dahlén, G.; Lyamuya, E.; Lagergård, T. Specificity of antibodies directed against the cytolethal distending toxin of Haemophilus ducreyi in patients with chancroid. Microb. Pathog. 2003, 35, 133–137. [Google Scholar] [CrossRef]
- Xynogala, I.; Volgina, A.; DiRienzo, J.M.; Korostoff, J. Evaluation of the humoral immune response to the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans Y4 in subjects with localized aggressive periodontitis. Oral Microbiol. Immunol. 2009, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ando-Suguimoto, E.S.; da Silva, M.P.; Kawamoto, D.; Chen, C.; DiRienzo, J.M.; Mayer, M.P.A. The cytolethal distending toxin of Aggregatibacter actinomycetemcomitans inhibits macrophage phagocytosis and subverts cytokine production. Cytokine 2014, 66, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.A.; Tarkowski, A.; Thelestam, M.; Lagergård, T. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb. Pathog. 2001, 30, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Del Bel Belluz, L.; Guidi, R.; Pateras, I.S.; Levi, L.; Mihaljevic, B.; Rouf, S.F.; Wrande, M.; Candela, M.; Turroni, S.; Nastasi, C.; et al. The Typhoid Toxin Promotes Host Survival and the Establishment of a Persistent Asymptomatic Infection. PLoS Pathog. 2016, 12, e1005528. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Findik, A.; Ica, T.; Onuk, E.E.; Percin, D.; Kevenk, T.O.; Ciftci, A. Molecular typing and CDT genes prevalence of Campylobacter jejuni isolates from various sources. Trop. Anim. Health Prod. 2011, 43, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Prasad, K.N.; Sinha, S.; Husain, N. Differences in virulence attributes between cytolethal distending toxin positive and negative Campylobacter jejuni strains. J. Med. Microbiol. 2008, 57, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, E.; Dzierzanowska-Fangrat, K.; Jozwiak, P.; Popowski, J.; Korsak, D.; Dzierzanowska, D. Prevalence of potential virulence markers in Polish Campylobacter jejuni and Campylobacter coli isolates obtained from hospitalized children and from chicken carcasses. J. Med. Microbiol. 2005, 54, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Rogers, A.B.; Whary, M.T.; Ge, Z.; Taylor, N.S.; Xu, S.; Horwitz, B.H.; Erdman, S.E. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 2004, 72, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Purdy, D.; Leach, S.A.; Hodgson, A.E.; Buswell, C.M.; Henderson, I.; McALPINE, K. Characterisation of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J. Med. Microbiol. 2000, 49, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.B.; Taylor, C.T.; Parkos, C.A.; Colgan, S.P. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: Role of intracellular cAMP. J. Cell. Physiol. 1998, 176, 76–84. [Google Scholar] [CrossRef]
- Lewis, D.A.; Mitjà, O. Haemophilus ducreyi: From sexually transmitted infection to skin ulcer pathogen. Curr. Opin. Infect. Dis. 2016, 29, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.J.; Svensson, L.A.; Cope, L.D.; Latimer, J.L.; Hansen, E.J.; Ahlman, K.; Bayat-Turk, J.; Klamer, D.; Lagergård, T. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J. Med. Microbiol. 2001, 50, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.K.; Latimer, J.L.; Lumbley, S.R.; Ward, C.K.; Cope, L.D.; Lagergard, T.; Hansen, E.J. Characterization of a Haemophilus ducreyi Mutant Deficient in Expression of Cytolethal Distending Toxin. Infect. Immun. 1999, 67, 3900–3908. [Google Scholar] [PubMed]
- Young, R.S.; Fortney, K.R.; Gelfanova, V.; Phillips, C.L.; Katz, B.P.; Hood, A.F.; Latimer, J.L.; Munson, R.S.; Hansen, E.J.; Spinola, S.M. Expression of Cytolethal Distending Toxin and Hemolysin Is Not Required for Pustule Formation by Haemophilus ducreyi in Human Volunteers. Infect. Immun. 2001, 69, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Wising, C.; Mölne, L.; Jonsson, I.-M.; Ahlman, K.; Lagergård, T. The cytolethal distending toxin of Haemophilus ducreyi aggravates dermal lesions in a rabbit model of chancroid. Microbes Infect. Inst. Pasteur 2005, 7, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Yang, M.; Geng, Y.; Chen, H.; Xu, Y.; Sun, Y. Prevalence and distribution of Aggregatibacter actinomycetemcomitans and its cdtB gene in subgingival plaque of Chinese periodontitis patients. BMC Oral Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Mínguez, M.; Pousa, X.; Herrera, D.; Blasi, A.; Sánchez, M.C.; León, R.; Sanz, M. Characterization and serotype distribution of Aggregatibacter actinomycetemcomitans isolated from a population of periodontitis patients in Spain. Arch. Oral Biol. 2014, 59, 1359–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mínguez, M.; Ennibi, O.K.; Pousa, X.; Lakhdar, L.; Abdellaoui, L.; Sánchez, M.; Sanz, M.; Herrera, D. Characterization of A. actinomycetemcomitans strains in subgingival samples from periodontitis subjects in Morocco. Clin. Oral Investig. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Song, K.-P.; Ong, G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J. Periodontal Res. 2002, 37, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Vitale, L.A.; Welham, D.A. Immune suppression induced by Actinobacillus actinomycetemcomitans: Effects on immunoglobulin production by human B cells. Infect. Immun. 1990, 58, 3856–3862. [Google Scholar] [PubMed]
- Akifusa, S.; Poole, S.; Lewthwaite, J.; Henderson, B.; Nair, S.P. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 2001, 69, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S.; Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis, Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, e615486. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Somoza-Martín, J.M.; Barros-Angueira, F.; Rey, J.M.G.; García-García, A. RANK/RANKL/OPG role in distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.M.W.; Gillespie, M.T. Modulation of osteoclast formation. Biochem. Biophys. Res. Commun. 2005, 328, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Brage, M.; Lagergård, T.; Johansson, A. Cytolethal distending toxin upregulates RANKL expression in Jurkat T-cells. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2008, 116, 499–506. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Johansson, A.; Wang, Y.; Chen, C.; Kalfas, S.; Lerner, U.H. The Cytolethal Distending Toxin Induces Receptor Activator of NF-κB Ligand Expression in Human Gingival Fibroblasts and Periodontal Ligament Cells. Infect. Immun. 2005, 73, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Damek-Poprawa, M.; Korostoff, J.; Gill, R.; DiRienzo, J.M. Cell Junction Remodeling in Gingival Tissue Exposed to a Microbial Toxin. J. Dent. Res. 2013, 92, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Miyauchi, M.; Tsuruda, K.; Takata, T.; Sugai, M. Topical application of Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces cell cycle arrest in the rat gingival epithelium in vivo. J. Periodontal Res. 2011, 46, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Guidi, R.; Guerra, L.; Levi, L.; Stenerlöw, B.; Fox, J.G.; Josenhans, C.; Masucci, M.G.; Frisan, T. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cell. Microbiol. 2013, 15, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Sautès-Fridman, C.; Fridman, W.H. The immune response in cancer: From immunology to pathology to immunotherapy. Virchows. Arch. Int. J. Pathol. 2015, 467, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Dewhirst, F.E.; Tully, J.G.; Paster, B.J.; Yan, L.; Taylor, N.S.; Collins, M.J.; Gorelick, P.L.; Ward, J.M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 1994, 32, 1238–1245. [Google Scholar] [PubMed]
- Liyanage, N.P.M.; Dassanayake, R.P.; Kuszynski, C.A.; Duhamel, G.E. Contribution of Helicobacter hepaticus cytolethal distending toxin subunits to human epithelial cell cycle arrest and apoptotic death in vitro. Helicobacter 2013, 18, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Rogers, A.B.; Feng, Y.; Lee, A.; Xu, S.; Taylor, N.S.; Fox, J.G. Bacterial cytolethal distending toxin promotes the development of dysplasia in a model of microbially induced hepatocarcinogenesis. Cell. Microbiol. 2007, 9, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar]
- Graillot, V.; Dormoy, I.; Dupuy, J.; Shay, J.W.; Huc, L.; Mirey, G.; Vignard, J. Genotoxicity of Cytolethal Distending Toxin (CDT) on Isogenic Human Colorectal Cell Lines: Potential Promoting Effects for Colorectal Carcinogenesis. Front. Cell. Infect. Microbiol. 2016, 34. [Google Scholar] [CrossRef] [PubMed]



| Select CDT-Producing Species | Pathology | Possible Contribution of CDT |
|---|---|---|
| Aggregatibacter actinomycetemcomitans | Aggressive periodontal disease | Aggressiveness of disease |
| Campylobacter jejuni | Inflammatory diarrhea | Prolongation of symptoms, Persistence of infection |
| Colorectal cancer-associated Escherichia coli | Colorectal cancer | Potential promotion of cancer initiation or progression, Potential promotion of carcinogenesis |
| Haemophilus ducreyi | Chancroid lesions | Development of ulcers, Persistence of lesions |
| Helicobacter hepaticus | Hepatitis, hepatocarcinoma (in mice) | Contribution to carcinogenesis (inflammation-induced carcinogenesis) |
| Salmonella enterica serovar Typhi | Diarrhea | Prolongation of symptoms, Persistence of infection |
| Shigella dysenteriae/boydii | Diarrhea | Role to be defined |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faïs, T.; Delmas, J.; Serres, A.; Bonnet, R.; Dalmasso, G. Impact of CDT Toxin on Human Diseases. Toxins 2016, 8, 220. https://doi.org/10.3390/toxins8070220
Faïs T, Delmas J, Serres A, Bonnet R, Dalmasso G. Impact of CDT Toxin on Human Diseases. Toxins. 2016; 8(7):220. https://doi.org/10.3390/toxins8070220
Chicago/Turabian StyleFaïs, Tiphanie, Julien Delmas, Arnaud Serres, Richard Bonnet, and Guillaume Dalmasso. 2016. "Impact of CDT Toxin on Human Diseases" Toxins 8, no. 7: 220. https://doi.org/10.3390/toxins8070220
APA StyleFaïs, T., Delmas, J., Serres, A., Bonnet, R., & Dalmasso, G. (2016). Impact of CDT Toxin on Human Diseases. Toxins, 8(7), 220. https://doi.org/10.3390/toxins8070220