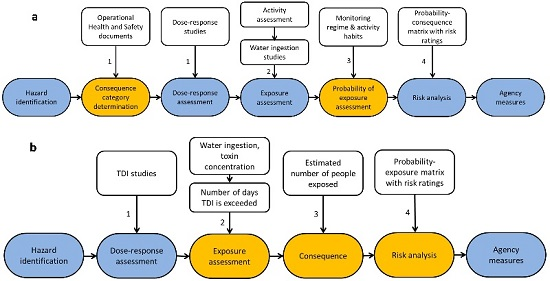
1. Introduction
Water pollution is a major threat to human health and one of humanity’s biggest challenges [
1,
2]. One approach to reduce the risk that pollution events pose to the public is the use of alert level frameworks that are based on thresholds for health-harmful pollution levels [
3,
4]. Their implementation is triggered by the detection of a pollution event. Alert level frameworks differentiate between the seriousness of consequences that arise from a range of exposures, allowing managers to progressively adapt their response by restricting access, passive monitoring or active treatment (e.g., [
5,
6,
7,
8,
9,
10]). Their focus is to facilitate response management in that they serve to identify actions to be put into place to reduce the acute risks posed by the immediate, detected occurrence of pollution. Through these alert level frameworks, agencies are generally well prepared to respond to such discrete pollution events. However, their effectiveness is based on the fact that the pollution is detected and reported in the first place.
With many systems not receiving any regular monitoring, pollution events often go undetected. This potentially puts the public at risk. The probability of being exposed to a hazard that is undetected depends on the monitoring frequency of a system [
11], which is governed by the allocation of limited financial resources to agencies and environmental projects [
12]. However, any suggestion to increase monitoring efforts is unrealistic as it is not economically viable for most agencies. Instead, understanding and identifying the risk a system poses to the public in regards to a pollutant of interest, assuming that not all pollution levels are detected due to infrequent monitoring would be an important step towards developing meaningful and sustainable management approaches.
The overall objective of this study was to develop toxicological risk assessment models for acute and chronic exposure to pollutants which incorporate the probabilities that the public comes into contact with undetected pollution events. These models can be used to assess the overall risk a system poses to the public with regards to a chosen pollutant and inform authorities on strategic management, including monitoring strategies and implementation of measures such as banning of recreational use to reduce the risk to the public.
We illustrate the development of the models using microcystins, which are toxins produced by aquatic cyanobacteria, as the pollutant of interest. To assess the applicability of the developed models, we then applied them to data collected by Sinang, Reichwaldt and Ghadouani [
13], who monitored microcystin concentrations in urban lakes. Microcystins pose a major threat to human health when present in drinking water, recreational waterbodies and in water used for crop irrigation [
14,
15]. They can cause acute poisoning and, through chronic exposure, liver cancer [
5,
16,
17,
18,
19,
20]. As cyanobacterial bloom frequency and toxin occurrence will likely increase in the future due to climate change and increasing nutrient pollution of waterbodies [
21,
22,
23,
24] and because microcystins have been shown to persist in lake sediment and accumulate in the food web [
25,
26] it is essential to develop management strategies that are informed by risk assessment to ensure the safety of the public. While the models are developed for acute and chronic risk assessment of microcystins they are general and can be adapted to any pollutant by replacing respective pollutant thresholds.
4. Discussion
The models developed here serve to assess the potential acute and chronic risks that systems pose to the health of the public with respect to a pollutant of interest. In comparison with existing frameworks which are emergency responses to single occurring pollution events [
31], our models integrate the probabilities that the public comes into contact with undetected pollution events and as such present an important risk management tool for agencies.
Applying the acute and chronic models to historical monitoring data demonstrated its potential to determine the expected health outcomes of recreational activities related to waterbodies containing microcystin-LR. As the frameworks are based on toxicological studies and risk management guidelines, it delivers an objective risk assessment using the information that is available for a pollutant. Integrating these frameworks into management practises can support agencies and councils in identifying lakes that pose a high threat to people and can further serve as a decision making tool towards identifying the appropriate control measures to lower the risk. Our model identifies the risk to people, if a system is not monitored at all. The results suggest that if a lake is used by the public only infrequently for swimming and if a low chronic risk is acceptable, then having no monitoring or very infrequent monitoring might be appropriate.
Our study highlights the significant impact of controls on acute risk ratings. Controls for protecting the public from pollutants, including cyanobacterial blooms in lakes are usually in the form of warning or regulatory signs [
9] or alert warnings in local newspapers. In addition, risk education has been identified as an important way to increase awareness and change behaviour [
46] and could help increase the number of people heeding the warning signs. However, these controls do not avoid the human fragility of oversight [
47] and research indicates that even the best signs are not obeyed by 100% of those seeing them [
48]. Guidelines for standard signs for water safety are available [
9] but their effectiveness in having people obey them depends on motivational and social circumstances highlighting the complexity of developing effective controls to reduce the exposure with the hazard [
48]. The risk rating of a lake can also be decreased by reducing the number of pollution events. This can be achieved by preventing contamination from outside sources including fining polluters or by adopting lake management techniques that are known to decrease the presence of the pollutant within the lake (e.g., [
49,
50]).
The accuracy and outcomes of the models developed here strongly depend on the availability of toxicological studies that identified relevant concentration levels of interest and on the risk appetite of the authorities, which will affect the risk ratings. Due to the relatively limited number of toxicological studies into microcystin-LR and studies into the ingestion of water for risk assessment from different activities, there are several significant sources of uncertainty in the results in our case study, including: the uncertainty in the toxicological dose dependent response to obtain defined consequences during exposure, and the uncertainty in the amount of water ingested per activity per day. As acute incidents may be triggered by a single, high-exposure event, it is necessary to estimate the maximum volume of water that an individual might ingest, rather than use estimates of the amount of water regularly ingested [
31,
35,
37,
51,
52]. In addition, the accuracy of the risk calculated using these frameworks strongly depends on the availability of true long-term data of a system. Ideal are direct measurements of the pollutant at high frequencies, in particular when pollutant concentrations are highly variable over short time periods due to pollutant production and degradation [
13,
53]. In practise, such data sets are rare. In the case for managing cyanobacteria, waterbodies are often analysed monthly or fortnightly and the limited resources allow only the quantification of cell numbers of potentially toxic cyanobacterial species. Converting those cell numbers into toxin concentrations adds another uncertainty that greatly affects the outcome of the framework. These sources of uncertainty are addressed in guidelines and risk assessment frameworks, including the ones presented here by incorporating high uncertainty factors, assuming worst case scenarios in relation to ingestion volumes, and factors to convert cell counts to toxin concentrations (e.g., [
6]). As such, the risk ratings identified here can be considered as highly conservative.
The reliability and accuracy of risk models depends strongly on the quality of the data on critical doses and the identification of potential short term (acute) and long-term effects on humans [
54]. For microcystins, as seen in our case study, the absence of a toxicological study into the time-dependent response of mice to doses exceeding the TDI (chronic) and the lack of a study unifying the dose-dependent acute consequences to exposure resulted in the need to extract data from multiple studies to characterise these responses. Further, while the ingestion of water during swimming has been well studied [
37,
51,
52], we could only find one study for people participating in non-immersive water sports other than swimming [
38]. Despite this, we successfully demonstrated that the models performed well and were able to identify different risk levels for the lakes, depending on the recreational activity level. Using the models for other pollutants, for which better knowledge of doses and acute and long term effects exists, will increase their accuracy.
The frameworks developed here can be adapted to any pollutant that shows a dose-dependent response to exposure (e.g.,
E. coli [
55]), including gaseous pollutants like hydrogen sulfide (H
2S) and greenhouse gases [
56], because only the dose dependent response components of the risk assessment frameworks are specific to the toxicology of microcystin-LR. Its application might be especially important in the wastewater sector due to an increased reuse of wastewater for various purposes. Further, wastewater contains more pollutants than lakes, many of which are biological hazards. With several agencies acknowledging that the potential risk from chemicals is lower than from water borne biological hazards (cyanobacteria) [
9,
32], we suggest to further test its applicability for a wider range of contaminants, water bodies and activities.
5. Conclusions
The proposed risk models succeeded in characterising risk in a way which is both objective and relevant to decision makers. The acute and chronic models were both able to characterise the risk and differentiate between proposed acceptable and unacceptable risks, and can be used to identify by how much the probability of exposure has to be decreased to lower the risk to an acceptable level. This will enable management authorities to identify appropriate control measures to be put in place, such as warning or regulatory signs, alert warnings in local newspapers, and increased monitoring and fining of polluters. We suggest broadening the application of these models by investigating their application to other contaminants and to waterbodies with multiple constituents of concern.
The accuracy of the results of the risk assessment models depend upon precise input values from toxicological studies of pollutants (here: microcystin-LR) and studies into ingestion volumes during specified activities. This uncertainty is intensified by the absence of any toxicological study that covers the entire spectrum of injuries possible through acute microcystin-LR exposure. Therefore in order to conduct a dose-dependent response assessment, dosage values must be extracted from different studies with varying methodologies, increasing the level of uncertainty in the assessment. We therefore call for more standardised toxicological studies on the health effects of contaminates, including cyanobacterial toxins, which would improve the accuracy of the results delivered by the developed models.