miR-541 Contributes to Microcystin-LR-Induced Reproductive Toxicity through Regulating the Expression of p15 in Mice
Abstract
:1. Introduction
2. Results
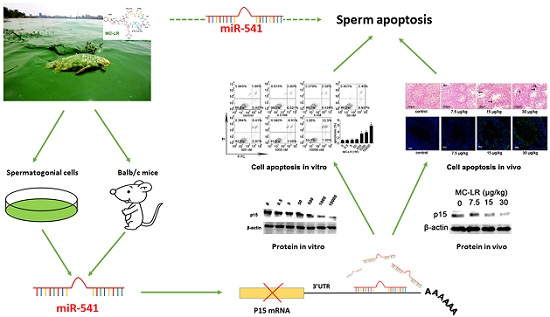
2.1. The Expression of p15 Is Regulated by miR-541 in GC-1 Cells
2.2. p15 May Regulate Apoptosis via the MDM2-p53 Pathway in GC-1 Cells
2.3. Inhibition of miR-541 Protects GC-1 Cells from MC-LR-Induced Cell Death in Vitro
2.4. MC-LR Regulates the Expression of miR-541 and p15 in Vivo
2.5. Inhibition of miR-541 Protects GC-1 Cells from MC-LR-Induced Cell Death in Vivo
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. Quantitative RT-PCR
4.6. Western Blot
4.7. FDA/PI Staining
4.8. TUNEL Staining
4.9. Flow Cytometry Analysis.
4.10. Dual-luciferase Reporter Assay
4.11. Histopathological Evaluation
4.12. Immunohistochemistry
4.13. Statistical Analysis.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Mougin, C.; Bouaicha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Mulvenna, V.; Dale, K.; Priestly, B.; Mueller, U.; Humpage, A.; Shaw, G.; Allinson, G.; Falconer, I. Health risk assessment for cyanobacterial toxins in seafood. Int. J. Environ. Res. Public Health 2012, 9, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; Paatero, G.I.; Meriluoto, J.A.; Codd, G.A.; Kass, G.E.; Nicotera, P.; Orrenius, S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp. Cell Res. 1989, 185, 86–100. [Google Scholar] [CrossRef]
- Van Apeldoorn, M.E.; van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P. Tissue distributions and seasonal dynamics of the hepatotoxic microcystins-LR and -RR in two freshwater shrimps, Palaemon modestus and Macrobrachium nipponensis, from a large shallow, eutrophic lake of the subtropical China. Toxicon 2005, 45, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, S.J.; Schmid, D.; Blom, J.F.; Ernst, B.; Dietrich, D.R. Analytical and functional characterization of microcystins [Asp3]MC-RR and [Asp3,Dhb7]MC-RR: Consequences for risk assessment? Environ. Sci. Technol. 2007, 41, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Carmichael, W.W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 1994, 32, 1495–1507. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Steinman, A.D. Microcystin-LR induced thyroid dysfunction and metabolic disorders in mice. Toxicology 2015, 328, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kist, L.W.; Rosemberg, D.B.; Pereira, T.C.; de Azevedo, M.B.; Richetti, S.K.; de Castro Leão, J.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Microcystin-LR acute exposure increases AChE activity via transcriptional ache activation in zebrafish (Danio rerio) brain. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.P.; Davis, M.A.; Ryan, T.P.; Searfoss, G.H.; Hooser, S.B.; Pathol, T.; Davis, M.A.; Ryan, T.P.; Searfoss, G.H.; Hooser, S.B. Hepatic gene expression changes in mice associated with prolonged sublethal microcystin exposure. Toxicol. Pathol. 2007, 35, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, A.; Zorc-Pleskovic, R.; Petrovic, D.; Zorc, M.; Suput, D. Microcystin-LR induces alterations in heart muscle. Folia Biol. 2006, 52, 116–118. [Google Scholar] [PubMed]
- Zegura, B.; Zajc, I.; Lah, T.T.; Filipič, M. Patterns of microcystin-LR induced alteration of the expression of genes involved in response to DNA damage and apoptosis. Toxicon 2008, 51, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xu, L.; Zhang, X.; Wang, Y.; Meng, X.; Miao, A.; Yang, L. Endoplasmic reticulum stress in murine liver and kidney exposed to microcystin-LR. Toxicon 2010, 56, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, S.; Zhou, F.; Wen, S.; Chen, F.; Han, X. Reproductive toxicity on female mice induced by microcystin-LR. Environ. Toxicol. Pharmacol. 2014, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Li, Y.; Han, X. Decline of sperm quality and testicular function in male mice during chronic low-dose exposure to microcystin-LR. Reprod. Toxicol. 2011, 31, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Yuan, M.; Xiang, Z.; Han, X. In vivo study on the effects of microcystin-LR on the apoptosis, proliferation and differentiation of rat testicular spermatogenic cells of male rats injected i.p. with toxins. J. Toxicol. Sci. 2013, 38, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, J.; Wu, J.; Han, X. The toxic effects of microcystin-LR on rat spermatogonia in vitro. Toxicol. Lett. 2012, 212, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Judice, C.C.; Bourgard, C.; Kayano, A.C.A.V.; Albrecht, L.; Costa, F.T.M. MicroRNAs in the Host-Apicomplexan Parasites Interactions: A Review of Immunopathological Aspects. Front. Cell. Infect. Microbiol. 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, Q.; Xie, P. Analysis of microRNA expression in embryonic developmental toxicity induced by MC-RR. PLoS ONE 2011, 6, e22676. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qin, W.; Zhang, H.; Wang, Y.; Dou, H.; Yu, D.; Ding, Y.; Yang, L.; Wang, Y. Alterations in microRNA expression linked to microcystin-LR-induced tumorigenicity in human WRL-68 Cells. Mutat. Res. 2012, 743, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiang, Z.; Li, D.; Han, X. Regulation of microcystin-LR-induced toxicity in mouse spermatogonia by miR-96. Environ. Sci. Technol. 2014, 48, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sharma, R.B.; Nwosu, B.U.; Alonso, L.C. Islet biology, the CDKN2A/B locus and type 2 diabetes risk. Diabetologia 2016, 59, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Kundu, R.K.; Cheng, H.; Maegdefessel, L.; Toh, R.; Ahn, G.O.; Ali, Z.A.; Anderson, D.R.; et al. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Lin, L.; Hong, H. Protein profiles in zebrafish (Danio rerio) brains exposed to chronic microcystin-LR. Chemosphere 2010, 81, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.D.; Henry, T.B.; Twiner, M.J.; Gouffon, J.S.; McPherson, J.T.; Boyer, G.L.; Sayler, G.S.; Wilhelm, S.W. Global gene expression profiling in larval zebrafish exposed to microcystin-LR and microcystis reveals endocrine disrupting effects of Cyanobacteria. Environ. Sci. Technol. 2011, 45, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Dumasia, K.; Kumar, A.; Deshpande, S.; Sonawane, S.; Balasinor, N.H. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Mol. Cell. Endocrinol. 2016, 428, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, K.; Henriksén, K.; Hirvonen, V.; Rannikko, S.; Salo, J.; Parvinen, M.; Dunkel, L. Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J. Clin. Endocrinol. Metab. 1997, 82, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yuan, M.; Song, Y.; Sun, F.; Han, X. MC-LR Exposure leads to subfertility of female mice and induces oxidative stress in granulosa cells. Toxins 2015, 7, 5212–5223. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.F.; Hou, C.C.; Yang, W.X. Small non-coding RNAs and their associated proteins in spermatogenesis. Gene 2016, 578, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Xie, P.; Li, H.Y.; Hao, L.; Xiong, Q.; Qiu, T. Involment of p53, Bax, and Bcl-2 pathway in microcystins-induced apoptosis in rat testis. Environ. Toxicol. 2011, 26, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Fladmark, K.E.; Brustugun, O.T.; Hovland, R.; Boe, R.; Gjertsen, B.T.; Zhivotovsky, B.; Døskeland, S.O. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 1999, 6, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Svircev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J. Environ. Sci. Health C 2010, 28, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, K.; Xu, Y.; Gao, Y.; Li, C.; Wang, R.; Chen, L. The role of microRNA-26a in human cancer progression and clinical application. Tumour Biol. 2016, 37, 7095–7108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Weng, Z.H.; Tsang, K.S.; Tsang, L.L.; Chan, H.C.; Jiang, X.H. MycN Is Critical for the maintenance of human embryonic stem cell-derived neural crest stem cells. PLoS ONE 2016, 11, e0148062. [Google Scholar]
- Inoue, S.; Tomasini, R.; Rufini, A.; Elia, A.J.; Agostini, M.; Amelio, I.; Cescon, D.; Dinsdale, D.; Zhou, L.; Harris, I.S.; et al. TAp73 is required for spermatogenesis and the maintenance of male fertility. Proc. Natl. Acad. Sci. USA 2014, 111, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, J.J. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the light: The growing complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Menendez, D.; Inga, A.; Resnick, M.A. The expanding universe of p53 targets. Nat. Rev. Cancer 2009, 9, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wu, H.; Leng, R.P. UBE4B targets phosphorylated p53 at serines 15 and 392 for degradation. Oncotarget 2016, 7, 2823–2836. [Google Scholar] [PubMed]
- Ogawa, T.; Aréchaga, J.M.; Avarbock, M.R.; Brinster, R.L. Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 1997, 41, 111–122. [Google Scholar] [PubMed]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef] [PubMed]
- Aigner, A.; Ray, P.E.; Czubayko, F.; Wellstein, A. Immunolocalization of an FGF-binding protein reveals a widespread expression pattern during different stages of mouse embryo development. Histochem. Cell Biol. 2002, 117, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaske, S.; Krasteva, G.; König, P.; Kummer, W.; Hofmann, T.; Gudermann, T.; Chubanov, V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007, 8, 49. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, L.; Chen, X.; Xiang, Z.; Li, D.; Han, X. miR-541 Contributes to Microcystin-LR-Induced Reproductive Toxicity through Regulating the Expression of p15 in Mice. Toxins 2016, 8, 260. https://doi.org/10.3390/toxins8090260
Meng X, Zhang L, Chen X, Xiang Z, Li D, Han X. miR-541 Contributes to Microcystin-LR-Induced Reproductive Toxicity through Regulating the Expression of p15 in Mice. Toxins. 2016; 8(9):260. https://doi.org/10.3390/toxins8090260
Chicago/Turabian StyleMeng, Xiannan, Ling Zhang, Xiang Chen, Zou Xiang, Dongmei Li, and Xiaodong Han. 2016. "miR-541 Contributes to Microcystin-LR-Induced Reproductive Toxicity through Regulating the Expression of p15 in Mice" Toxins 8, no. 9: 260. https://doi.org/10.3390/toxins8090260
APA StyleMeng, X., Zhang, L., Chen, X., Xiang, Z., Li, D., & Han, X. (2016). miR-541 Contributes to Microcystin-LR-Induced Reproductive Toxicity through Regulating the Expression of p15 in Mice. Toxins, 8(9), 260. https://doi.org/10.3390/toxins8090260