The Contents of Ustiloxins A and B along with Their Distribution in Rice False Smut Balls
Abstract
:1. Introduction
2. Results
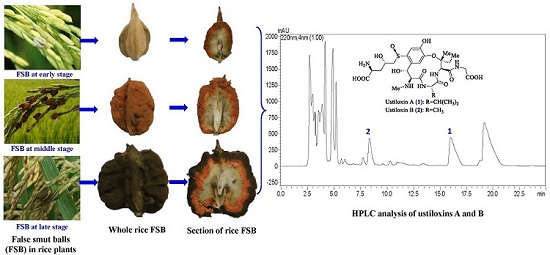
2.1. The Contents of Ustiloxins A and B in Matured Rice FSBs
2.2. Distribution of Ustiloxins A and B in Rice FSBs at Different Maturity Stages
3. Discussion
4. Conclusions
5. Experimental Section
5.1. General Experimental Procedures
5.2. HPLC Analysis of Ustiloxins A and B
5.3. Detection of Ustiloxins A and B in Rice FSBs from Different Areas and Harvest Years
5.4. Detection of Ustiloxins A and B in Rice FSBs at Different Maturity Stages
5.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, J.; Yang, J.; Wang, Y.-Q.; Li, G.-B.; Li, Y.; Huang, F.; Wang, W.-M. Current understanding on Villosiclava virens, a unique flower-infecting fungus causing rice false smut disease. Mol. Plant Pathol. 2016, 17. [Google Scholar] [CrossRef]
- Tanaka, E.; Ashizawa, T.; Sonoda, R.; Tanaka, C. Villosiclava virens gen. nov., comb. nov., teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon 2008, 106, 491–501. [Google Scholar]
- Ashizawa, T.; Takahashi, M.; Arai, M.; Arie, T. Rice false smut pathogen, Ustilaginoidea virens, invades through small gap at the apex of a rice spikelet before heading. J. Gen. Plant Pathol. 2012, 78, 255–259. [Google Scholar] [CrossRef]
- Hu, M.; Luo, L.; Wang, S.; Liu, Y.; Li, J. Infection processes of Ustilaginoidea virens during artificial inoculation of rice panicles. Eur. J. Plant Pathol. 2014, 139, 66–77. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, J.; Hu, D.; Yong, M.; Xu, Y.; He, L. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol. 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaoka, K.; Kobayashi, T.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994, 47, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Izumiyama, N.; Ohtsubo, K.; Koiso, Y.; Iwasaki, S.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. “Lupinosis”-like lesions in mice caused by ustiloxin, produced by Ustilaginoidea virens: A morphological study. Nat. Toxins 1994, 2, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, S.; Shan, T.; Wang, P.; Sun, W.; Chen, Z.; Wang, S. Chemistry and biology of mycotoxins from rice false smut pathogen. In Mycotoxins: Properties, Applications and Hazards; Melborn, B.J., Greene, J.C., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 109–130. [Google Scholar]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-naphtho-γ-pyrones from fungi and their bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, W.; Meng, J.; Wang, A.; Wang, X.; Tian, J.; Fu, X.; Dai, J.; Liu, Y.; Lai, D.; et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Sun, W.; Mao, Z.; Xu, D.; Wang, X.; Lu, S.; Lai, D.; Liu, Y.; Zhou, L.; Zhang, G. Main ustilaginoidins and their distribution in rice false smut balls. Toxins 2015, 7, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dong, X.; Xu, D.; Meng, J.; Fu, X.; Wang, X.; Lai, D.; Zhou, L.; Liu, Y. Preparative separation of main ustilaginoidins from rice false smut balls by high-speed counter-current chromatography. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Matsumoto, Y.; Uchihara, T.; Morimoto, K. High-performance liquid chromatographic determination of ustiloxin A in forage rice silage. J. Vet. Med. Sci. 2009, 71, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Sun, W.; Wang, X.; Fu, X.; Sun, W.; Zhou, L. Purification of ustiloxins A and B from rice false smut balls by macroporous resins. Molecules 2013, 18, 8181–8199. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Sun, W.; Liu, H.; Gao, S.; Lu, S.; Wang, M.; Sun, W.; Chen, Z.; Wang, S.; Zhou, L. Determination and analysis of ustiloxins A and B by LC-ESI-MS and HPLC in false smut balls of rice. Int. J. Mol. Sci. 2012, 13, 11275–11287. [Google Scholar] [CrossRef] [PubMed]
- Luduena, R.F.; Roach, M.C.; Prasad, V.; Banerjee, M.; Koiso, Y.; Li, Y.; Iwasaki, S. Interaction of ustiloxin A with bovine brain tubulin. Biochem. Pharmacol. 1994, 47, 1593–1599. [Google Scholar] [CrossRef]
- Li, Y.; Koiso, Y.; Kobayashi, H.; Hashimoto, Y.; Iwasaki, S. Ustiloxins, new antimitotic cyclic peptides: Interaction with porcine brain tubulin. Biochem. Pharmacol. 1995, 49, 1367–1372. [Google Scholar] [CrossRef]
- Morisaki, N.; Mitsui, Y.; Yamashita, Y.; Koiso, Y.; Shirai, R.; Hashimoto, Y.; Iwasaki, S. Synthesis and anti-tubulin activity of ustiloxin D derivatives. J. Antibiot. 1998, 51, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Koiso, Y.; Natori, M.; Iwasaki, S.; Sato, S.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxin: A phytotoxin and a mycotoxin from false smuth balls on rice panicles. Tetrahedron Lett. 1992, 33, 4157–4160. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Cartwright, R.D.; Sciumbato, G.L. Ustilaginoidea virens infection of rice in Arkansas: Toxicity of false smut galls, their extracts and the ustiloxin fraction. Am. J. Plant Sci. 2014, 5, 3166–3176. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Cui, Y.; Wang, A.; Lai, D.; Liu, Y.; Li, Q.; Wang, B.; Zhou, L. A monoclonal antibody-based enzyme-linked immunosorbent assay for detection of ustiloxin A in rice false smut balls and rice samples. Food Chem. 2015, 181, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, A.; Wang, X.; Lin, F.; He, L.; Lai, D.; Liu, Y.; Li, Q.; Zhou, L.; Wang, B. Development of a monoclonal antibody-based icELISA for the detection of ustiloxin B in rice false smut balls and rice grains. Toxins 2015, 7, 3481–3496. [Google Scholar] [CrossRef] [PubMed]




| Collection Area (Longitude and Latitude) and Time of Rice FSBs | Average Weight of Each FSB (mg) | Ustiloxin Content (µg/g) | Ratio of the Contents of Ustiloxins A and B | ||
|---|---|---|---|---|---|
| Ustiloxin A | Ustiloxin B | Total | |||
| Anqing (116.6° E, 30.6° N), Anhui; Aug. 2012 | 88.1 ± 17.1 c,d,e | 884.6 ± 12.2 c | 411.0 ± 15.7 b | 1295.6 ± 27.8 c | 2.152 |
| Chengdu (104.1° E, 30.57° N), Sichuan; Sep. 2014 | 92.0 ± 15.4 c | 584.1 ± 16.4 e | 139.0 ± 2.1 f | 723.0 ± 14.4 g | 4.202 |
| Donggang (124.2° E, 39.9° N), Liaoning; Nov. 2010 | 87.2 ± 14.9 c,d,e | 369.3 ± 28.4 h | 57.8 ± 11.3 g | 427.1 ± 24.8 h | 6.389 |
| Donggang (124.2° E, 39.9° N), Liaoning; Nov. 2011 | 86.7 ± 11.6 c,d,e | 333.1 ± 9.8 h | 31.2 ± 14.3 h | 364.3 ± 15.6 i | 10.676 |
| Donggang (124.2° E, 39.9° N), Liaoning; Nov. 2014 | 73.1 ± 6.0 e | 617.1 ± 1.3 e | 234.4 ± 13.3 c,d | 851.5 ± 13.2 e | 2.633 |
| Guilin (110.3° E, 25.3° N), Guangxi; Sep. 2015 | 74.6 ± 7.3 d,e | 600.5 ± 9.0 e | 194.1 ± 0.4 e | 794.6 ± 8.7 f | 3.095 |
| Hanshou (112.0° E, 28.9° N), Hunan; Sep. 2013 | 95.8 ± 9.8 b,c | 583.9 ± 14.0 e | 229.8 ± 6.3 d | 813.7 ± 20.0 e,f | 2.541 |
| Hanshou (112.0° E, 28.9° N), Hunan; China; Sep. 2015 | 94.1 ± 15.1 b,c | 292.5 ± 33.8 i | 39.7 ± 6.4 g,h | 332.2 ± 40.1 i | 7.368 |
| Jianou (118.3°E, 27.1°N), Fujian; Nov. 2012 | 89.3 ± 11.5 c,d | 466.5 ± 30.0 g | 251.0 ± 5.6 c | 717.4 ± 33.3 g | 1.859 |
| Linyi (118.4° E, 35.1° N), Shandong; Oct. 2011 | 142.0 ± 18.8 a | 1023.2 ± 27.7 b | 516.2 ± 11.1 a | 1539.4 ± 17.5 b | 1.982 |
| Linyi (118.4° E, 35.1° N), Shandong; Oct. 2012 | 144.1 ± 13.9 a | 696.5 ± 15.7 d | 248.3 ± 13.7 c,d | 944.9 ± 29.4 d | 2.805 |
| Linyi (118.4° E, 35.1° N), Shandong; Oct. 2013 | 107.3 ± 5.3 b | 1064.3 ± 14.3 a | 518.0 ± 2.9 a | 1582.3 ± 13.3 a | 2.055 |
| Qionglai (103.5° E, 30.4° N), Sichuan; Sep. 2012 | 73.4 ± 13.5 e | 528.1 ± 26.7 f | 155.4 ± 17.7 f | 683.5 ± 42.9 g | 3.398 |
| Zhangjiagang (120.6° E, 31.9° N), Jiangsu; Nov. 2015 | 99.4 ± 21.2 b,c | 723.8 ± 35.7 d | 232.5 ± 13.5 c,d | 956.3 ± 22.7 d | 3.113 |
| Part of Rice FSBs | Average Weight in Each FSB (mg) | Ustiloxin Content (µg/g) | Total Ustiloxin Yield (µg/Each Part or Ball) | ||
|---|---|---|---|---|---|
| Ustiloxin A | Ustiloxin B | Total | |||
| Early maturity stage | |||||
| Out layer | nd | nd | nd | nd | nd |
| Middle layer | 13.3 ± 2.3 g,h | 2324.0 ± 58.1 a | 1304.3 ± 23.0 a | 3628.3 ± 81.0 a | 48.3 |
| Inner part | 15.6 ± 1.5 f,g | 742.8 ± 21.8 c | 226.9 ± 9.8 c | 969.7 ± 31.3 c | 15.1 |
| Glume | 4.0 ± 0.5 i | 64.0 ± 5.8 i | nd | 64.0 ± 5.8 h | 0.3 |
| Whole ball | 32.9 ± 2.4 c | 1294.7 ± 80.0 b | 632.0 ± 52.6 b | 1926.7 ± 132.6 b | 63.4 |
| Middle maturity stage | |||||
| Out layer | 8.7 ± 2.2 h,i | 354.8 ± 15.1 f,g | 35.5 ± 3.2 e,f | 390.2 ± 18.2 f | 3.4 |
| Middle layer | 20.9 ± 2.9 e,f | 493.2 ± 10.8 e | 72.6 ± 3.2 e | 565.8 ± 13.8 e | 11.8 |
| Inner part | 26.0 ± 4.1 d,e | 335.3 ± 2.4 f,g | 18.7 ± 2.5 f | 353.9 ± 3.3 f,g | 9.2 |
| Glume | 3.6 ± 0.4 i | nd | nd | nd | nd |
| Whole ball | 59.2 ± 4.4 b | 373.3 ± 8.4 f | 39.0 ± 2.0 e,f | 410.0 ± 14.0 f | 24.3 |
| Late maturity stage | |||||
| Out layer | 29.6 ± 7.7 c,d | 254.2 ± 20.7 h | 28.1 ± 7.9 f | 282.2 ± 28.5 g | 8.4 |
| Middle layer | 26.5 ± 9.0 d,e | 669.5 ± 9.1 d | 111.7 ± 5.5 d | 781.1 ± 14.3 d | 20.7 |
| Inner part | 34.3 ± 5.5 c | 74.5 ± 10.0 i | nd | 74.5 ± 10.0 h | 2.6 |
| Glume | 3.7 ± 0.9 i | nd | nd | nd | nd |
| Whole ball | 94.1 ± 15.1 a | 292.5 ± 33.8 g,h | 39.7 ± 6.4 e,f | 332.2 ± 40.1 f,g | 31.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fu, X.; Lin, F.; Sun, W.; Meng, J.; Wang, A.; Lai, D.; Zhou, L.; Liu, Y. The Contents of Ustiloxins A and B along with Their Distribution in Rice False Smut Balls. Toxins 2016, 8, 262. https://doi.org/10.3390/toxins8090262
Wang X, Fu X, Lin F, Sun W, Meng J, Wang A, Lai D, Zhou L, Liu Y. The Contents of Ustiloxins A and B along with Their Distribution in Rice False Smut Balls. Toxins. 2016; 8(9):262. https://doi.org/10.3390/toxins8090262
Chicago/Turabian StyleWang, Xiaohan, Xiaoxiang Fu, Fengke Lin, Weibo Sun, Jiajia Meng, Ali Wang, Daowan Lai, Ligang Zhou, and Yang Liu. 2016. "The Contents of Ustiloxins A and B along with Their Distribution in Rice False Smut Balls" Toxins 8, no. 9: 262. https://doi.org/10.3390/toxins8090262
APA StyleWang, X., Fu, X., Lin, F., Sun, W., Meng, J., Wang, A., Lai, D., Zhou, L., & Liu, Y. (2016). The Contents of Ustiloxins A and B along with Their Distribution in Rice False Smut Balls. Toxins, 8(9), 262. https://doi.org/10.3390/toxins8090262