Integrated On-Chip 3D Vascular Network Culture under Hypoxia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Construction
2.2. Device Characterization
2.3. Cell Culture and Vascular Network Development under Normoxic–Hypoxic Transition
3. Results
3.1. Characterization of O2 and CO2 Levels
3.2. Network Formation in the Normoxic Condition
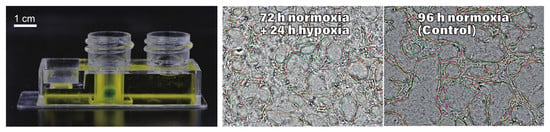
3.3. Network Formation and Development in the Normoxic–Hypoxic Transition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bae, H.; Puranik, A.S.; Gauvin, R.; Edalat, F.; Carrillo-Conde, B.; Peppas, N.A.; Khademhosseini, A. Building vascular networks. Sci. Transl. Med. 2012, 4, 160ps23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, A.; Albers, H.J.; Passier, R.; Mummery, C.L.; Van Den Berg, A.; Orlova, V.V.; Van Der Meer, A.D. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2019, 140, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, S.; Ranga, A. Engineering Organoid Vascularization. Front. Bioeng. Biotechnol. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toussaint, O.; Weemaels, G.; Debacq-Chainiaux, F.; Scharffetter-Kochanek, K.; Wlaschek, M. Artefactual effects of oxygen on cell culture models of cellular senescence and stem cell biology. J. Cell. Physiol. 2011, 226, 315–321. [Google Scholar] [CrossRef]
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Yu, D.; Yan, X.; Wang, B.; Yu, Z.; Song, Y.; Sheng, L. Hypoxia destroys the microstructure of microtubules and causes dysfunction of endothelial cells via the PI3K/Stathmin1 pathway. Cell Biosci. 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Semba, H.; Takeda, N. The roles of hypoxia signaling in the pathogenesis of cardiovascular diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Eltzchig, H.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Rankin, E.B.; Giaccia, A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008, 15, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Baldea, I.; Teacoe, I.; Olteanu, D.E.; Vaida-Voievod, C.; Clichici, A.; Sirbu, A.; Filip, G.A.; Clichici, S. Effects of different hypoxia degrees on endothelial cell cultures-Time course study. Mech. Ageing Dev. 2018, 172, 45–50. [Google Scholar] [CrossRef]
- Wu, J.; Lei, Z.; Yu, J. Hypoxia induces autophagy in human vascular endothelial cells in a hypoxia-inducible factor 1dependent manner. Mol. Med. Rep. 2015, 11, 2677–2682. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 2004, 6, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Fathollahipour, S.; Patil, P.S.; Leipzig, N.D. Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells Tissues Organs 2018, 205, 350–371. [Google Scholar] [CrossRef]
- Hutton, D.L.; Grayson, W.L. Hypoxia inhibits de novo vascular assembly of adipose-derived stromal/stem cell populations, but promotes growth of preformed vessels. Tissue Eng. Part A 2016, 22, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, E.; Grayson, W.L. Assessing the Minimum Time-Period of Normoxic Preincubation for Stable Adipose Stromal Cell-Derived Vascular Networks. Cell Mol. Bioeng. 2018, 11, 471–481. [Google Scholar] [CrossRef]
- Byrne, M.B.; Leslie, M.T.; Gaskins, H.R.; Kenis, P.J.A. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol. 2014, 32, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Funamoto, K.; Zervantonakis, I.K.; Liu, Y.; Ochs, C.J.; Kim, C.; Kamm, R.D. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab. Chip 2012, 12, 4855–4863. [Google Scholar] [CrossRef] [Green Version]
- Bakmiwewa, S.M.; Heng, B.; Guillemin, G.J.; Ball, H.J.; Hunt, N.H. An effective, low-cost method for achieving and maintaining hypoxia during cell culture studies. Biotechniques 2015, 59, 223–224, 226, 228–229. [Google Scholar] [CrossRef]
- Skolimowski, M.; Nielsen, M.W.; Emnéus, J.; Molin, S.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab. Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; King, A.D.; Shih, H.C.; Peng, C.C.; Wu, C.Y.; Liao, W.H.; Tung, Y.C. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab. Chip 2011, 11, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bansal, T.; Pinelis, M.; Maharbiz, M.M. A microsystem for sensing and patterning oxidative microgradients during cell culture. Lab. Chip 2006, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Lockett, M.R.; Minn, K.T.; Simon, K.A.; Gilbert, K.; Hillier, S.; Newsome, D.; Li, H.; Hall, A.B.; Boucher, D.M.; et al. A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials 2015, 52, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Lee, T.A.; Ko, P.L.; Chiang, H.J.; Peng, C.C.; Tung, Y.C. Review of microfluidic cell culture devices for the control of gaseous microenvironments in vitro. J. Micromech. Microeng. 2018, 28. [Google Scholar] [CrossRef]
- Munoz-Sanchez, J.; Chanez-Cardenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef]
- Niu, N.; Li, Z.; Zhu, M.; Sun, H.; Yang, J.; Xu, S.; Zhao, W.; Song, R. Effects of nuclear respiratory factor1 on apoptosis and mitochondrial dysfunction induced by cobalt chloride in H9C2 cells. Mol. Med. Rep. 2019, 19, 2153–2163. [Google Scholar] [CrossRef] [Green Version]
- Reist, M.; Marshall, K.A.; Jenner, P.; Halliwell, B. Toxic effects of sulphite in combination with peroxynitrite on neuronal cells. J. Neurochem. 1998, 71, 2431–2438. [Google Scholar] [CrossRef]
- Vengellur, A.; Phillips, J.M.; Hogenesch, J.B.; LaPres, J.J. Gene expression profiling of hypoxia signaling in human hepatocellular carcinoma cells. Physiol. Genom. 2005, 22, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Takano, A.; Tanaka, M.; Futai, N. On-chip multi-gas incubation for microfluidic cell cultures under hypoxia. Biomicrofluidics 2014, 8, 061101. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, G. Angiogenesis Analyzer for ImageJ. Available online: http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ (accessed on 20 December 2019).
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab. Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab. Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481. [Google Scholar] [CrossRef] [PubMed]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Dyszkiewicz-Konwińska, M.; Piotrowska-Kempisty, H.; Zabel, M. Human Umbilical Vein Endothelial Cells (HUVECs) Co-Culture with Osteogenic Cells: From Molecular Communication to Engineering Prevascularised Bone Grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef] [Green Version]
- Costa-Almeida, R.; Granja, P.L.; Soares, R.; Guerreiro, S.G. Cellular strategies to promote vascularisation in tissue engineering applications. Eur. Cell Mater. 2014, 28, 51–66. [Google Scholar] [CrossRef]
- Grassi, L.; Alfonsi, R.; Francescangeli, F.; Signore, M.; De Angelis, M.L.; Addario, A.; Costantini, M.; Flex, E.; Ciolfi, A.; Pizzi, S.; et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.Y.; Liu, L.J.; Hu, Q.X. A Versatile Method for Fabricating Tissue Engineering Scaffolds with a Three-Dimensional Channel for Prevasculature Networks. ACS Appl. Mater. Interfaces 2016, 8, 25096–25103. [Google Scholar] [CrossRef]
- Metsala, O.; Kreutzer, J.; Hogel, H.; Miikkulainen, P.; Kallio, P.; Jaakkola, P.M. Transportable system enabling multiple irradiation studies under simultaneous hypoxia in vitro. Radiat. Oncol. 2018, 13, 220. [Google Scholar] [CrossRef]







| Condition | pH | Temperature (°C) | pCO2 (%) |
|---|---|---|---|
| Bicarbonate (Normoxia) | 7.13 ± 0.18 | 36.8 ± 0.06 | 5.19 ± 1.70 |
| Bicarbonate + Ascorbate (Hypoxia) | 7.11 ± 0.07 | 36.4 ± 0.05 | 5.22 ± 0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Suárez, M.Á.; Sekiguchi, T.; Takano, A.; Cañizares-Macías, M.d.P.; Futai, N. Integrated On-Chip 3D Vascular Network Culture under Hypoxia. Micromachines 2020, 11, 475. https://doi.org/10.3390/mi11050475
Olmedo-Suárez MÁ, Sekiguchi T, Takano A, Cañizares-Macías MdP, Futai N. Integrated On-Chip 3D Vascular Network Culture under Hypoxia. Micromachines. 2020; 11(5):475. https://doi.org/10.3390/mi11050475
Chicago/Turabian StyleOlmedo-Suárez, Miguel Ángel, Tomohiro Sekiguchi, Atsushi Takano, Maria del Pilar Cañizares-Macías, and Nobuyuki Futai. 2020. "Integrated On-Chip 3D Vascular Network Culture under Hypoxia" Micromachines 11, no. 5: 475. https://doi.org/10.3390/mi11050475
APA StyleOlmedo-Suárez, M. Á., Sekiguchi, T., Takano, A., Cañizares-Macías, M. d. P., & Futai, N. (2020). Integrated On-Chip 3D Vascular Network Culture under Hypoxia. Micromachines, 11(5), 475. https://doi.org/10.3390/mi11050475