A Preliminary Prototype High-Speed Feedback Control of an Artificial Cochlear Sensory Epithelium Mimicking Function of Outer Hair Cells
Abstract
:1. Introduction
2. Experimental Methods
2.1. Design and Fabrication of the Artificial Cochlear Sensory Epithelium
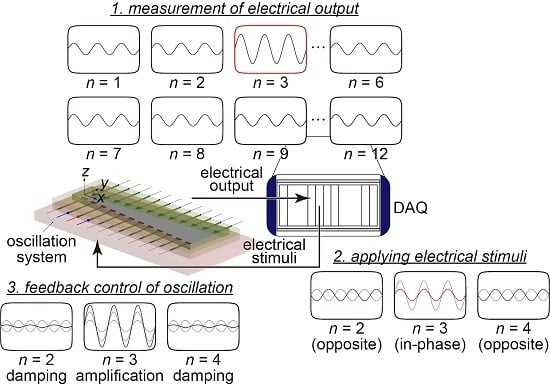
2.2. Experimental Setup for Measurements and Feedback Control
- Each electrode deposited on the artificial cochlear sensory epithelium was oscillated via the inverse piezoelectric effect by applying a local electrical stimulus to the control electrodes. The steady-state oscillation of for each electrode was measured by the LDV, and the frequency characteristics of the oscillation amplitude Z were analyzed to observe the frequency selectivity of the device, as described in Section 3.1.
- The displacement and the electrical output generated by the piezoelectric effect were simultaneously measured with external mechanical oscillations applied to the fixed trapezoidal boundary with a constant acceleration amplitude . The frequency characteristics of the amplitudes Z and were evaluated, as described in Section 3.2.
- Based on the output signals from the device, the oscillations of the artificial cochlear sensory epithelium were controlled, as described in Section 3.4. Testing this feedback control approach was the main purpose of this study.
2.3. Noteworthy Improvement of Feedback Control
3. Experimental Results and Discussion
3.1. Frequency Selectivity of the Artificial Cochlear Sensory Epithelium Measured with the Inverse Piezoelecric Effect
3.2. Measurement of Output Electrical Signal by Applying the External Mechanical Oscillations
3.3. Challenges in the Fabrication Process and Oscillation Measurements for the Continuous Trapezoidal Membrane
3.4. Feedback Control of the Oscillation in a Trapezoidal Membrane
4. Conclusions
- An improved artificial cochlear sensory epithelium was developed on the basis of our previous device [29]. The device consists of a bimorph PVDF piezoelectric membrane to achieve frequency selectivity from the piezoelectric output and generate a higher voltage. A mechanical oscillation system that can apply a constant acceleration to the boundary of the trapezoidal membrane was used to measure the electrical signals from the device.
- The frequency characteristics of the oscillation amplitude and the electrical output were systematically and simultaneously measured, and it was found that they the showed peaks at the corresponding resonance frequency for each electrode. Thus, the measurement of the electrical output can be used to identify the resonance position.
- The adhesion of the PVDF membrane to the jig strongly affected the accuracy and reproducibility of the data, which was qualitatively discussed based on the linear theory of beam oscillation. When the boundary conditions were not fixed ends but simple supports, the resonance frequencies and the piezoelectric output generated by the strain were reduced. The validity of the model was qualitatively confirmed by comparing the theoretical values with the experimental results.
- Feedback control of the membrane oscillation was performed. The resonance position was determined by the amplitude of the electrical output, and the corresponding electrode was electrically stimulated via a nearby control electrode. The oscillation amplitude at the resonance position was successfully amplified in this way, and the sharpness of the vibration peak was improved in experimental tests. In comparison with the previously developed feedback principle [29], the speed of feedback control was drastically improved, with the control time reduced by approximately 99%.
Author Contributions
Funding
Conflicts of Interest
Appendix A. Implantation of the Artificial Cochlear Sensory Eepithelium in Future Clinical Use

References
- Robles, L.; Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. rev. 2001, 81, 1305–1352. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N. Schuknecht’s Pathology of the Ear; PMPH-USA: Shelton, CT, USA, 2010. [Google Scholar]
- Guinan, J.J., Jr.; Salt, A.; Cheatham, M.A. Progress in cochlear physiology after Békésy. Hear. Res. 2012, 293, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichenbach, T.; Hudspeth, A. The physics of hearing: Fluid mechanics and the active process of the inner ear. Rep. Prog. Phys. 2014, 77, 076601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Koike, T.; Wang, J.; Sienz, H.; Meredith, R. Finite element analysis of the middle ear transfer functions and related pathologies. Med. Eng. Phys. 2009, 31, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Torikai, H.; Nishigami, T. An artificial chaotic spiking neuron inspired by spiral ganglion cell: Paralleled spike encoding, theoretical analysis, and electronic circuit implementation. Neural Netw. 2009, 22, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A. Making an effort to listen: Mechanical amplification in the ear. Neuron 2008, 59, 530–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Olson, E.S. Detection of cochlear amplification and its activation. Biophys. J. 2013, 105, 1067–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, T.; Nin, F.; Choi, S.; Muramatsu, S.; Sawamura, S.; Ogata, G.; Sato, M.P.; Doi, K.; Doi, K.; Tsuji, T.; et al. Characterisation of the static offset in the travelling wave in the cochlear basal turn. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Böhnke, F.; Stark, T. Three-dimensional representation of the human cochlea using micro-computed tomography data: Presenting an anatomical model for further numerical calculations. Acta Oto-Laryngol. 2012, 132, 603–613. [Google Scholar] [CrossRef]
- Pelliccia, P.; Venail, F.; Bonafé, A.; Makeieff, M.; Iannetti, G.; Bartolomeo, M.; Mondain, M. Cochlea size variability and implications in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 42. [Google Scholar]
- Warchol, M.E.; Lambert, P.R.; Goldstein, B.J.; Forge, A.; Corwin, J.T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 1993, 259, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.G. Trends in cochlear implants. Trends Amplif. 2004, 8, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, R.M.; Do, B.S.T. Future of implantable auditory devices. Otolaryngol. Clin. N. Am. 2019, 52, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.S.; Dorman, M.F. Cochlear implants: A remarkable past and a brilliant future. Hear. Res. 2008, 242, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.S.; Dorman, M.F. Cochlear implants: Current designs and future possibilities. J. Rehabil. Res. Dev. 2008, 45, 695–730. [Google Scholar] [CrossRef]
- Crathorne, L.; Bond, M.; Cooper, C.; Elston, J.; Weiner, G.; Taylor, R.; Stein, K. A systematic review of the effectiveness and cost-effectiveness of bilateral multichannel cochlear implants in adults with severe-to-profound hearing loss. Clin. Otolaryngol. 2012, 37, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.B.; Webster, M. Effects of neonatal conductive hearing loss on brain stem auditory nuclei. Ann. Otol. Rhinol. Laryngol. 1979, 88, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Nakagawa, T.; Ono, K.; Ogita, H.; Sakamoto, T.; Yamamoto, N.; Okita, K.; Yamanaka, S.; Ito, J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport 2009, 20, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Skerleva, D.; Kitajiri, S.i.; Sakamoto, T.; Yamamoto, N.; Ito, J.; Nakagawa, T. Limited hair cell induction from human induced pluripotent stem cells using a simple stepwise method. Neurosci. Lett. 2015, 599, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Ohnishi, H.; Skerleva, D.; Sakamoto, T.; Yamamoto, N.; Hotta, A.; Ito, J.; Nakagawa, T. Transplantation of neurons derived from human iPS cells cultured on collagen matrix into guinea-pig cochleae. J. Tissue Eng. Regen. Med. 2017, 11, 1766–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintaku, H.; Nakagawa, T.; Kitagawa, D.; Tanujaya, H.; Kawano, S.; Ito, J. Development of piezoelectric acoustic sensor with frequency selectivity for artificial cochlea. Sens. Actuators A Phys. 2010, 158, 183–192. [Google Scholar] [CrossRef]
- Jung, Y.; Kwak, J.H.; Lee, Y.H.; Kim, W.D.; Hur, S. Development of a multi-channel piezoelectric acoustic sensor based on an artificial basilar membrane. Sensors 2014, 14, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chung, J.; Hwang, G.T.; Jeong, C.K.; Jung, Y.; Kwak, J.H.; Kang, H.; Byun, M.; Kim, W.D.; Hur, S.; et al. Flexible inorganic piezoelectric acoustic nanosensors for biomimetic artificial hair cells. Adv. Funct. Mater. 2014, 24, 6914–6921. [Google Scholar] [CrossRef]
- Jung, Y.; Kwak, J.H.; Kang, H.; Kim, W.D.; Hur, S. Mechanical and electrical characterization of piezoelectric artificial cochlear device and biocompatible packaging. Sensors 2015, 15, 18851–18864. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Jung, Y.; Song, K.; Hur, S. Fabrication of Si3N4-Based Artificial Basilar Membrane with ZnO Nanopillar Using MEMS Process. J. Sens. 2017, 2017, 1308217. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, J.S.; Kim, G.W. A novel frequency selectivity approach based on travelling wave propagation in mechanoluminescence basilar membrane for artificial cochlea. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Liu, J.; Zhang, Y.; Liu, J.; Zhai, J. Design of bionic cochlear basilar membrane acoustic sensor for frequency selectivity based on film triboelectric nanogenerator. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, T.; Nakayama, A.; Yamazaki, H.; Kawano, S. Artificial cochlear sensory epithelium with functions of outer hair cells mimicked using feedback electrical stimuli. Micromachines 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.; Kim, S.; Sly, D.J.; O’leary, S.J.; Choi, H. MEMS piezoelectric artificial basilar membrane with passive frequency selectivity for short pulse width signal modulation. Sens. Actuators A Phys. 2013, 203, 6–10. [Google Scholar] [CrossRef]
- Jang, J.; Jang, J.H.; Choi, H. MEMS flexible artificial basilar membrane fabricated from piezoelectric aluminum nitride on an SU-8 substrate. J. Micromech. Microeng. 2017, 27, 075006. [Google Scholar] [CrossRef]
- Jeon, H.; Jang, J.; Kim, S.; Choi, H. Characterization of a Piezoelectric AlN Beam Array in Air and Fluid for an Artificial Basilar Membrane. Electron. Mater. Lett. 2018, 14, 101–111. [Google Scholar] [CrossRef]
- Park, S.; Guan, X.; Kim, Y.; Creighton, F.P.X.; Wei, E.; Kymissis, I.; Nakajima, H.H.; Olson, E.S. PVDF-based piezoelectric microphone for sound detection inside the cochlea: Toward totally implantable cochlear implants. Trends Hear. 2018, 22, 2331216518774450. [Google Scholar] [CrossRef] [PubMed]
- Ilik, B.; Koyuncuoğlu, A.; Şardan-Sukas, Ö.; Külah, H. Thin film piezoelectric acoustic transducer for fully implantable cochlear implants. Sens. Actuators A Phys. 2018, 280, 38–46. [Google Scholar] [CrossRef]
- Tsuji, T.; Imada, Y.; Yamazaki, H.; Kawano, S. Simultaneous measurement of the oscillation characteristics and electrical voltage output of an artificial cochlear sensory epithelium immersed in a liquid: Theory and experiment. Sens. Actuators A Phys. 2019, 295, 414–427. [Google Scholar] [CrossRef]
- Shintaku, H.; Tateno, T.; Tsuchioka, N.; Tanujaya, H.; Nakagawa, T.; Ito, J.; Kawano, S. Culturing neurons on MEMS fabricated P (VDF-TrFE) films for implantable artificial cochlea. J. Biomech. Sci. Eng. 2010, 5, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Inaoka, T.; Shintaku, H.; Nakagawa, T.; Kawano, S.; Ogita, H.; Sakamoto, T.; Hamanishi, S.; Wada, H.; Ito, J. Piezoelectric materials mimic the function of the cochlear sensory epithelium. Proc. Natl. Acad. Sci. USA 2011, 108, 18390–18395. [Google Scholar] [CrossRef] [Green Version]
- Shintaku, H.; Inaoka, T.; Nakagawa, T.; Kawano, S.; Ito, J. Electrically Evoked Auditory Brainstem Response by Using Bionic Auditory Membrane in Guinea Pigs. J. Biomech. Sci. Eng. 2013, 8, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Lee, J.; Woo, S.; Sly, D.J.; Campbell, L.J.; Cho, J.H.; O’Leary, S.J.; Park, M.H.; Han, S.; Choi, J.W.; et al. A microelectromechanical system artificial basilar membrane based on a piezoelectric cantilever array and its characterization using an animal model. Sci. Rep. 2015, 5, 12447. [Google Scholar] [CrossRef]
- Zhao, C.; Knisely, K.E.; Colesa, D.J.; Pfingst, B.E.; Raphael, Y.; Grosh, K. Voltage readout from a piezoelectric intracochlear acoustic transducer implanted in a living guinea pig. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tona, Y.; Inaoka, T.; Ito, J.; Kawano, S.; Nakagawa, T. Development of an electrode for the artificial cochlear sensory epithelium. Hear. Res. 2015, 330, 106–112. [Google Scholar] [CrossRef]
- Joyce, B.S.; Tarazaga, P.A. Mimicking the cochlear amplifier in a cantilever beam using nonlinear velocity feedback control. Smart Mater. Struct. 2014, 23, 075019. [Google Scholar] [CrossRef]
- Joyce, B.S.; Tarazaga, P.A. Developing an active artificial hair cell using nonlinear feedback control. Smart Mater. Struct. 2015, 24, 094004. [Google Scholar] [CrossRef]
- Joyce, B.S.; Tarazaga, P.A. A study of active artificial hair cell models inspired by outer hair cell somatic motility. J. Intell. Mater. Syst. Struct. 2017, 28, 811–823. [Google Scholar] [CrossRef]
- Davaria, S.; Malladi, V.V.S.; Motaharibidgoli, S.; Tarazaga, P.A. Cochlear amplifier inspired two-channel active artificial hair cells. Mech. Syst. Signal Process. 2019, 129, 568–589. [Google Scholar] [CrossRef]
- Lighthill, J. Energy flow in the cochlea. J. Fluid Mech. 1981, 106, 149–213. [Google Scholar] [CrossRef]
- Steele, C.R.; Lim, K.M. Cochlear model with three-dimensional fluid, inner sulcus and feed-forward mechanism. Audiol. Neurotol. 1999, 4, 197–203. [Google Scholar] [CrossRef]
- White, R.D.; Grosh, K. Microengineered hydromechanical cochlear model. Proc. Natl. Acad. Sci. USA 2005, 102, 1296–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Cohen, H.I.; Bifano, T.G.; Castle, J.; Fortin, J.; Kapusta, C.; Mountain, D.C.; Zosuls, A.; Hubbard, A.E. A hydromechanical biomimetic cochlea: Experiments and models. J. Acoust. Soc. Am. 2006, 119, 394–405. [Google Scholar] [CrossRef] [PubMed]
- The Chemical Society of Japan. Handbook of Chemistry: Pure Chemistry, 4th ed.; Maruzen Publishing Co., Ltd.: Tokyo, Japan, 1993. [Google Scholar]
- Liu, Y.W.; Neely, S.T. Outer hair cell electromechanical properties in a nonlinear piezoelectric model. J. Acoust. Soc. Am. 2009, 126, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Nin, F.; Reichenbach, T.; Fisher, J.A.; Hudspeth, A. Contribution of active hair-bundle motility to nonlinear amplification in the mammalian cochlea. Proc. Natl. Acad. Sci. USA 2012, 109, 21076–21080. [Google Scholar] [CrossRef] [Green Version]
- Saremi, A.; Beutelmann, R.; Dietz, M.; Ashida, G.; Kretzberg, J.; Verhulst, S. A comparative study of seven human cochlear filter models. J. Acoust. Soc. Am. 2016, 140, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Ito, J.; Nakagawa, T.; Shintaku, H. Artificial sensory epithelium. US Patent 9,566,428, 14 February 2017. [Google Scholar]
- Mercier, P.P.; Lysaght, A.C.; Bandyopadhyay, S.; Chandrakasan, A.P.; Stankovic, K.M. Energy extraction from the biologic battery in the inner ear. Nat. Biotechnol. 2012, 30, 1240–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Process | Solution | Conditions |
|---|---|---|
| Spin coating | AZ5214-E | 500 rpm, 5 s |
| slope, 5 s | ||
| 3000 rpm, 30 s | ||
| Prebake | – | 70, 15 min |
| UV light exposure | – | 6 |
| Development and etching | AZ300MIF | approx. 8 min |
| Rinse | ethanol | 2 min |
| Electrode | 1 | 3 | 4 | 5 | 7 | 10 |
|---|---|---|---|---|---|---|
| Length [mm] | 4.24 | 4.88 | 5.20 | 5.52 | 6.16 | 7.12 |
| Resonant frequency [kHz] | 10.5 | 7.80 | 6.40 | 5.40 | 4.30 | 3.50 |
| [] | 189 | 186 | 173 | 165 | 167 | 163 |
| Electrode | 3 | 4 | 5 | 7 |
|---|---|---|---|---|
| without feedback control | 1.51 | 1.45 | 0.978 | 1.09 |
| with feedback control | 1.63 | 1.41 | 1.40 | 1.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, H.; Yamanaka, D.; Kawano, S. A Preliminary Prototype High-Speed Feedback Control of an Artificial Cochlear Sensory Epithelium Mimicking Function of Outer Hair Cells. Micromachines 2020, 11, 644. https://doi.org/10.3390/mi11070644
Yamazaki H, Yamanaka D, Kawano S. A Preliminary Prototype High-Speed Feedback Control of an Artificial Cochlear Sensory Epithelium Mimicking Function of Outer Hair Cells. Micromachines. 2020; 11(7):644. https://doi.org/10.3390/mi11070644
Chicago/Turabian StyleYamazaki, Hiroki, Dan Yamanaka, and Satoyuki Kawano. 2020. "A Preliminary Prototype High-Speed Feedback Control of an Artificial Cochlear Sensory Epithelium Mimicking Function of Outer Hair Cells" Micromachines 11, no. 7: 644. https://doi.org/10.3390/mi11070644
APA StyleYamazaki, H., Yamanaka, D., & Kawano, S. (2020). A Preliminary Prototype High-Speed Feedback Control of an Artificial Cochlear Sensory Epithelium Mimicking Function of Outer Hair Cells. Micromachines, 11(7), 644. https://doi.org/10.3390/mi11070644