Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization
Abstract
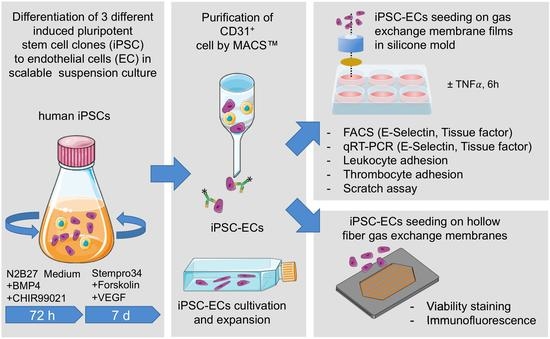
:1. Introduction
2. Materials and Methods
2.1. General Remarks
2.2. Generation and Cultivation of Human iPSCs
2.3. Generation and Purification of iPSC-Derived ECs
2.4. Cultivation of ECs on Tissue Culture Plastic and PMP Membranes
2.5. Cultivation of ECs on PMP HFM
2.6. Cryo-Sectioning and Immunofluorescence Staining of Seeded HFM
2.7. TNFα Assay
2.8. Flow Cytometry
2.9. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Leukocyte Adhesion Assay
2.11. Thrombocyte Adhesion Assay
2.12. Real-Time Reendothelialization Assay
2.13. Statistical Analysis
3. Results
3.1. Efficient Endothelialization of HFM
3.2. iPSC-ECs on PMP Membrane Are in a Non-Activated State While Remaining Physiologic Responsive to TNFα Stimulation
3.3. Leukocyte Adhesion Assay Confirms the Non-Inflammatory Status of iPSC-EC Monolayers
3.4. iPSC-ECs Improve HFM Hemocompatibility
3.5. Regenerative Capacity of PMP Membrane Seeded iPSC-ECs to Reendothelialize Scratched Monolayer Areas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 4 July 2021).
- ISHLT Research and Data. Available online: https://ishlt.org/research-data/registries (accessed on 12 June 2021).
- Pham, T.; Combes, A.; Rozé, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. Extracorporeal Membrane Oxygenation for Pandemic Influenza A(H1N1)–Induced Acute Respiratory Distress Syn-drome. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef]
- Shaefi, S.; The STOP-COVID Investigators; Brenner, S.K.; Gupta, S.; O’Gara, B.P.; Krajewski, M.L.; Charytan, D.M.; Chaudhry, S.; Mirza, S.H.; Peev, V.; et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021, 47, 208–221. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fan-ning, J.J.; et al. Extracorporeal Membrane Oxygenation Support in COVID-19: An International Cohort Study of the Extra-corporeal Life Support Organization Registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Kumar, A.; Keshavamurthy, S.; Abraham, J.G.; Toyoda, Y. Massive Air Embolism Caused by a Central Venous Catheter during Extracorporeal Membrane Oxygenation. J. Extra-Corpor. Technol. 2019, 51, 9–11. [Google Scholar] [PubMed]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015, 7, 320–326. [Google Scholar] [PubMed]
- Dalton, H.J.; Garcia-Filion, P.; Holubkov, R.; Moler, F.W.; Shanley, T.; Heidemann, S.; Meert, K.; Berg, R.A.; Berger, J.; Carcillo, J.; et al. Association of Bleeding and Thrombosis with Outcome in Extracorporeal Life Support. Pediatr. Crit. Care Med. 2015, 16, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2018, 44, 020–029. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The Inflammatory Response to Extracorporeal Mem-brane Oxygenation (ECMO): A Review of the Pathophysiology. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lehle, K.; Philipp, A.; Gleich, O.; Holzamer, A.; Müller, T.; Bein, T.; Schmid, C. Efficiency in Extracorporeal Membrane Oxygenation—Cellular Deposits on Polymethypentene Membranes Increase Resistance to Blood Flow and Reduce Gas Exchange Capacity. ASAIO J. 2008, 54, 612–617. [Google Scholar] [CrossRef]
- Li, Z.; Nguyen, B.L.; Cheng, Y.C.; Xue, J.; MacLaren, G.; Yap, C.H. Durable, flexible, superhydrophobic and blood-repelling surfaces for use in medical blood pumps. J. Mater. Chem. B 2018, 6, 6225–6233. [Google Scholar] [CrossRef]
- Wu, X.; Liew, Y.; Mai, C.-W.; Then, Y. Potential of Superhydrophobic Surface for Blood-Contacting Medical Devices. Int. J. Mol. Sci. 2021, 22, 3341. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.C.; Waterhouse, A.; Hicks-Berthet, J.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Obstals, F.; Vorobii, M.; Riedel, T.; de los Santos Pereira, A.; Bruns, M.; Singh, S.; Rodriguez-Emmenegger, C. Improving Hemocompatibility of Membranes for Extracorporeal Membrane Oxygenators by Grafting Nonthrombogenic Polymer Brushes. Macromol. Biosci. 2018, 18, 1700359. [Google Scholar] [CrossRef]
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv. Compos. Hybrid Mater. 2021, 1–18. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, C.; Wiegmann, B.; Maurer, A.N.; Fischer, P.; Möller, L.; Martin, U.; Hilfiker, A.; Haverich, A.; Fischer, S. Reduced Thrombocyte Adhesion to Endothelialized Poly 4-Methyl-1-Pentene Gas Exchange Membranes—A First Step Toward Bioartificial Lung Development. Tissue Eng. Part A 2010, 16, 3043–3053. [Google Scholar] [CrossRef]
- Wiegmann, B.; Von Seggern, H.; Höffler, K.; Korossis, S.; Dipresa, D.; Pflaum, M.; Schmeckebier, S.; Seume, J.; Haverich, A. Developing a biohybrid lung–sufficient endothelialization of poly-4-methly-1-pentene gas exchange hollow-fiber membranes. J. Mech. Behav. Biomed. Mater. 2016, 60, 301–311. [Google Scholar] [CrossRef]
- Zwirner, U.; Höffler, K.; Pflaum, M.; Korossis, S.; Haverich, A.; Wiegmann, B. Identifying an optimal seeding protocol and endothelial cell substrate for biohybrid lung development. J. Tissue Eng. Regen. Med. 2018, 12, 2319–2330. [Google Scholar] [CrossRef]
- Schumer, E.; Höffler, K.; Kuehn, C.; Slaughter, M.; Haverich, A.; Wiegmann, B. In-Vitro evaluation of limitations and possibilities for the future use of intracorporeal gas exchangers placed in the upper lobe position. J. Artif. Organs 2017, 21, 68–75. [Google Scholar] [CrossRef]
- Pflaum, M.; Kühn-Kauffeldt, M.; Schmeckebier, S.; Dipresa, D.; Chauhan, K.; Wiegmann, B.; Haug, R.; Schein, J.; Haverich, A.; Korossis, S. Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for application in the bioartificial lung. Acta Biomater. 2017, 50, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Möller, L.; Hess, C.; Paleček, J.; Su, Y.; Haverich, A.; Kirschning, A.; Dräger, G. Towards a biocompatible artificial lung: Covalent functionalization of poly(4-methylpent-1-ene) (TPX) with cRGD pentapeptide. Beilstein J. Org. Chem. 2013, 9, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, C.G.; Dietrich, M.; Gromann, K.; Frese, J.; Krueger, S.; Sachweh, J.S.; Jockenhoevel, S. Fibronectin coating of oxygenator membranes enhances endothelial cell attachment. Biomed. Eng. Online 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polk, A.A.; Maul, T.; McKeel, D.T.; Snyder, T.; Lehocky, C.A.; Pitt, B.; Stolz, D.B.; Federspiel, W.J.; Wagner, W.R. A biohybrid artificial lung prototype with active mixing of endothelialized microporous hollow fibers. Biotechnol. Bioeng. 2010, 106, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Plein, T.; Thiebes, A.L.; Finocchiaro, N.; Hesselmann, F.; Schmitz-Rode, T.; Jockenhoevel, S.; Cornelissen, C.G. Towards a Biohybrid Lung Assist Device: N-Acetylcysteine Reduces Oxygen Toxicity and Changes Endothelial Cells’ Morphology. Cell. Mol. Bioeng. 2016, 10, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Finocchiaro, N.; Olszweski, S.; Arens, J.; Schmitz-Rode, T.; Sachweh, J.; Jockenhoevel, S.; Cornelissen, C.G. ENDOXY-Development of a Biomimetic Oxygenator-Test-Device. PLoS ONE 2015, 10, e0142961. [Google Scholar] [CrossRef]
- Menzel, S.; Finocchiaro, N.; Donay, C.; Thiebes, A.L.; Hesselmann, F.; Arens, J.; Djeljadini, S.; Wessling, M.; Schmitz-Rode, T.; Jockenhoevel, S.; et al. Towards a Biohybrid Lung: Endothelial Cells Promote Oxygen Transfer through Gas Permeable Membranes. BioMed Res. Int. 2017, 2017, 5258196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegmann, B.; Figueiredo, C.; Gras, C.; Pflaum, M.; Schmeckebier, S.; Korossis, S.; Haverich, A.; Blasczyk, R. Prevention of rejection of allogeneic endothelial cells in a biohybrid lung by silencing HLA-class I expression. Biomaterials 2014, 35, 8123–8133. [Google Scholar] [CrossRef]
- Corselli, M.; Parodi, A.; Mogni, M.; Sessarego, N.; Kunkl, A.; Dagna-Bricarelli, F.; Ibatici, A.; Pozzi, S.; Bacigalupo, A.; Fras-soni, F.; et al. Clinical Scale Ex Vivo Expansion of Cord Blood-Derived Outgrowth Endothelial Progenitor Cells Is Associat-ed with High Incidence of Karyotype Aberrations. Exp. Hematol. 2008, 36, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; Umbenhauer, D.R.; Hill, R.; Bradt, C.; Mueller, S.N.; Levine, E.M.; Nichols, W.W. Karyotypic and phenotypic changes during in vitro aging of human endothelial cells. J. Cell. Physiol. 1992, 150, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Buynak, E.B.; Bradt, C.; Hill, R.; Aronson, M.; Jarrell, B.E.; Mueller, S.N.; Levine, E.M. Cytogenetic evaluation of human endothelial cell cultures. J. Cell. Physiol. 1987, 132, 453–462. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Haase, A.; Olmer, R.; Schwanke, K.; Wunderlich, S.; Merkert, S.; Hess, C.; Zweigerdt, R.; Gruh, I.; Meyer, J.; Wagner, S.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood. Cell Stem Cell 2009, 5, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Gaignerie, A.; Lefort, N.; Rousselle, M.; Forest-Choquet, V.; Flippe, L.; Francois-Campion, V.; Girardeau, A.; Caillaud, A.; Chariau, C.; Francheteau, Q.; et al. Urine-Derived Cells Provide a Readily Accessible Cell Type for Feeder-Free MRNA Re-programming. Sci. Rep. 2018, 8, 14363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, A.; Göhring, G.; Martin, U. Generation of non-transgenic iPS cells from human cord blood CD34+ cells under animal component-free conditions. Stem Cell Res. 2017, 21, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Olmer, R.; Engels, L.; Usman, A.; Menke, S.; Malik, M.N.H.; Pessler, F.; Göhring, G.; Bornhorst, D.; Bolten, S.; Abdelilah-Seyfried, S.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture. Stem Cell Rep. 2018, 10, 1657–1672. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sürün, D.; Schneider, A.; Mircetic, J.; Neumann, K.; Lansing, F.; Paszkowski-Rogacz, M.; Hänchen, V.; Lee-Kirsch, M.A.; Buchholz, F. Efficient Generation and Correction of Mutations in Human iPS Cells Utilizing mRNAs of CRISPR Base Editors and Prime Editors. Genes 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Merkert, S.; Wunderlich, S.; Bednarski, C.; Beier, J.; Haase, A.; Dreyer, A.-K.; Schwanke, K.; Meyer, J.; Göhring, G.; Cathomen, T.; et al. Efficient Designer Nuclease-Based Homologous Recombination Enables Direct PCR Screening for Footprintless Targeted Human Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Loscalzo, J. Nitric Oxide Insufficiency, Platelet Activation, and Arterial Thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kader, K.N.; Akella, R.; Ziats, N.P.; Lakey, L.A.; Harasaki, H.; Ranieri, J.P.; Bellamkonda, R.V. eNOS-Overexpressing Endothelial Cells Inhibit Platelet Aggregation and Smooth Muscle Cell Proliferation in Vitro. Tissue Eng. 2000, 6, 241–251. [Google Scholar] [CrossRef]
- Okamoto, T.; Tanigami, H.; Suzuki, K.; Shimaoka, M. Thrombomodulin: A Bifunctional Modulator of Inflammation and Coagulation in Sepsis. Crit. Care Res. Pract. 2012, 2012, 614545. [Google Scholar] [CrossRef] [Green Version]
- Waugh, J.M.; Yuksel, E.; Li, J.; Kuo, M.D.; Kattash, M.; Saxena, R.; Geske, R.; Thung, S.N.; Shenaq, S.M.; Woo, S.L. Local Over-expression of Thrombomodulin for in Vivo Prevention of Arterial Thrombosis in a Rabbit Model. Circ. Res. 1999, 84, 84–92. [Google Scholar] [CrossRef]
- Haase, A.; Glienke, W.; Engels, L.; Göhring, G.; Esser, R.; Arseniev, L.; Martin, U. GMP-compatible manufacturing of three iPS cell lines from human peripheral blood. Stem Cell Res. 2019, 35, 101394. [Google Scholar] [CrossRef]
- Palecek, J.; Zweigerdt, R.; Olmer, R.; Martin, U.; Kirschning, A.; Dräger, G. A practical synthesis of Rho-Kinase inhibitor Y-27632 and fluoro derivatives and their evaluation in human pluripotent stem cells. Org. Biomol. Chem. 2011, 9, 5503–5510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.-X.; Gao, X.-H.; Pan, R.; Lu, D.; Dai, Y. A simple adhesion assay for studying interactions between platelets and endothelial cells in vitro. Cytotechnology 2010, 62, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaoing, L.R.; Post, A.D.; Lin, A.Y.; Tseng, H.; Moake, J.L.; Grande-Allen, K.J. Laminin Peptide-Immobilized Hydrogels Modulate Valve Endothelial Cell Hemostatic Regulation. PLoS ONE 2015, 10, e0130749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension Culture of Human Pluripotent Stem Cells in Controlled, Stirred Bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Post, A.; Wang, E.; Cosgriff-Hernandez, E. A Review of Integrin-Mediated Endothelial Cell Phenotype in the Design of Cardiovascular Devices. Ann. Biomed. Eng. 2018, 47, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Pober, J.S. The Cathepsin B Death Pathway Contributes to TNF Plus IFN-γ-Mediated Human Endothelial Injury. J. Immunol. 2005, 175, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubnow, M.; Philipp, A.; Foltan, M.; Enger, T.B.; Lunz, D.; Bein, T.; Haneya, A.; Schmid, C.; Riegger, G.; Müller, T.; et al. Technical Complications during Veno-Venous Extracorporeal Membrane Oxygenation and Their Relevance Predicting a System-Exchange—Retrospective Analysis of 265 Cases. PLoS ONE 2014, 9, e112316. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Seltsam, A.; Blasczyk, R. Class-, gene-, and group-specific HLA silencing by lentiviral shRNA delivery. J. Mol. Med. 2006, 84, 425–437. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pflaum, M.; Dahlmann, J.; Engels, L.; Naghilouy-Hidaji, H.; Adam, D.; Zöllner, J.; Otto, A.; Schmeckebier, S.; Martin, U.; Haverich, A.; et al. Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines 2021, 12, 981. https://doi.org/10.3390/mi12080981
Pflaum M, Dahlmann J, Engels L, Naghilouy-Hidaji H, Adam D, Zöllner J, Otto A, Schmeckebier S, Martin U, Haverich A, et al. Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines. 2021; 12(8):981. https://doi.org/10.3390/mi12080981
Chicago/Turabian StylePflaum, Michael, Julia Dahlmann, Lena Engels, Hossein Naghilouy-Hidaji, Denise Adam, Janina Zöllner, Annette Otto, Sabrina Schmeckebier, Ulrich Martin, Axel Haverich, and et al. 2021. "Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization" Micromachines 12, no. 8: 981. https://doi.org/10.3390/mi12080981
APA StylePflaum, M., Dahlmann, J., Engels, L., Naghilouy-Hidaji, H., Adam, D., Zöllner, J., Otto, A., Schmeckebier, S., Martin, U., Haverich, A., Olmer, R., & Wiegmann, B. (2021). Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines, 12(8), 981. https://doi.org/10.3390/mi12080981