In-Situ Integration of 3D C-MEMS Microelectrodes with Bipolar Exfoliated Graphene for Label-Free Electrochemical Cancer Biomarkers Aptasensor
Abstract
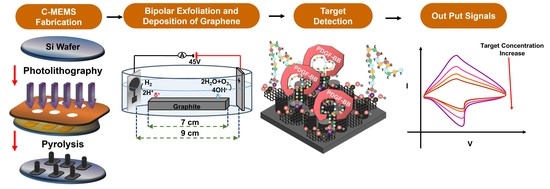
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. 3D C-MEMS Photolithography
2.4. Bipolar Exfoliation and Deposition of rGO on 3D C-MEMS Microelectrodes
2.5. Aptasensor Development
2.6. Electrochemical Characterization of Aptasensors
3. Results and Discussion
3.1. Material Characterization
3.2. Electrochemical Characterization
3.3. Sensing Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R. MEMS based cantilever biosensors for cancer detection using potential bio-markers present in VOCs: A survey. Microsyst. Technol. 2019, 25, 3253–3267. [Google Scholar]
- Kankaanpää, M.; Holma-Eriksson, M.; Kapanen, S.; Heitto, M.; Bergström, S.; Muukkonen, L.; Harjola, V.-P. Comparison of the use of comprehensive point-of-care test panel to conventional laboratory process in emergency department. BMC Emerg. Med. 2018, 18, 43. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Alam, F.; Khakpour, I.; Baboukani, A.R.; Pala, N.; Wang, C. Highly sensitive lactic acid biosensors based on photoresist derived carbon. IEEE Sens. J. 2020, 20, 8965–8972. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. A Review of electrochemical aptasensors for label-free cancer diagnosis. J. Electrochem. Soc. 2020, 167, 067511. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Future Oncol. 2005, 1, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Williams, P.J.; Niewolna, M.; Wang, Y.; Yoneda, T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002, 62, 917–923. [Google Scholar] [PubMed]
- Kawaguchi, J.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Yoshioka, T.; Itani, M.; Kozawa, O.; Moriwaki, H. UVC irradiation suppresses platelet-derived growth factor-BB-induced migration in human pancreatic cancer cells. Oncol. Rep. 2012, 27, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ye, H.; Liu, Z.; Xu, C.; Zhang, Z.; Liu, Y.; Sun, Y. Platelet-derived growth factor-BB accelerates prostate cancer growth by promoting the proliferation of mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 1510–1518. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Cobec, I.M.; Ceaușu, R.A.; Popescu, R.; Tudor, A.; Raica, M. Platelet derived growth factor BB: A “Must-have” therapeutic target “redivivus” in ovarian cancer. Cancer Genom. Proteom. 2016, 13, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.K.; Yang, Z.F.; Ho, D.W.; Ng, M.N.; Yeoh, G.C.; Poon, R.T.; Fan, S.T. An Akt/hypoxia-inducible factor-1α/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin. Cancer Res. 2009, 15, 3462–3471. [Google Scholar] [CrossRef] [Green Version]
- Penmatsa, V.; Ruslinda, A.R.; Beidaghi, M.; Kawarada, H.; Wang, C. Platelet-derived growth factor oncoprotein detection using three-dimensional carbon microarrays. Biosens. Bioelectron. 2013, 39, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Syahir, A.; Usui, K.; Tomizaki, K.-Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Rejeeth, C.; Xu, W.; Zhu, C.; Liu, X.; Wan, J.; Jiang, M.; Qian, K. Label-free electrochemical sensor for cd44 by ligand-protein interaction. Anal. Chem. 2019, 91, 7078–7085. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. Highly sensitive label-free electrochemical aptasensors based on photoresist derived carbon for cancer biomarker detection. Biosens. Bioelectron. 2020, 170, 112598. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Khakpour, I.; Alam, F.; Pala, N.; Wang, C. Novel application of electrochemical bipolar exfoliated graphene for highly sensitive disposable label-free cancer biomarker aptasensors. Nanoscale Adv. 2021, 3, 5948–5958. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Alam, F.; Jalal, A.H.; Forouzanfar, S.; Karabiyik, M.; Baboukani, A.R.; Pala, N. Flexible and linker-free enzymatic sensors based on zinc oxide nanoflakes for noninvasive L-lactate sensing in sweat. IEEE Sens. J. 2020, 20, 5102–5109. [Google Scholar] [CrossRef]
- Khorsandifard, M.; Jafari, K.; Sheikhaleh, A. A proposal for a novel surface-stress based BioMEMS sensor using an optical sensing system for highly sensitive diagnoses of Bio-particles. Sens. Imaging 2021, 22, 35. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Talebzadeh, N.; Zargari, S.; Veladi, H. The effect of microchannel width on mixing efficiency of microfluidic electroosmotic mixer. In Proceedings of the 2015 3rd RSI International Conference on Robotics and Mechatronics (ICROM), Tehran, Iran, 7–9 October 2015; pp. 629–634. [Google Scholar]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef]
- Cui, L.; Lu, M.; Li, Y.; Tang, B.; Zhang, C.-Y. A reusable ratiometric electrochemical biosensor on the basis of the binding of methylene blue to DNA with alternating AT base sequence for sensitive detection of adenosine. Biosens. Bioelectron. 2018, 102, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wei, S.-H.; Zhang, C.-Y. Construction of a robust entropy-driven DNA nanomachine for single-molecule detection of rare cancer cells. Anal. Chem. 2019, 91, 7505–7509. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, S.; Pala, N.; Madou, M.; Wang, C. Perspectives on C-MEMS and C-NEMS biotech applications. Biosens. Bioelectron. 2021, 180, 113119. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Madou, M. From MEMS to NEMS with carbon. Biosens. Bioelectron. 2005, 20, 2181–2187. [Google Scholar] [CrossRef]
- Ferrer-Argemi, L.; Aliabadi, E.S.; Cisquella-Serra, A.; Salazar, A.; Madou, M.; Lee, J. Size-dependent electrical and thermal conductivities of electro-mechanically-spun glassy carbon wires. Carbon 2018, 130, 87–93. [Google Scholar] [CrossRef]
- Piñón, M.V.; Benítez, B.C.; Pramanick, B.; Perez-Gonzalez, V.H.; Madou, M.J.; Martinez-Chapa, S.O.; Hwang, H. Direct current-induced breakdown to enhance reproducibility and performance of carbon-based interdigitated electrode arrays for AC electroosmotic micropumps. Sens. Actuators A Phys. 2017, 262, 10–17. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.R.; Okpowe, O.; Khakpour, I.; Safa, M.; Chen, C.; Wang, C. A high-energy aqueous on-chip lithium-ion capacitor based on interdigital 3D carbon microelectrode arrays. J. Power Sources 2020, 455, 227987. [Google Scholar] [CrossRef]
- Kumar, R. Materials selection approaches and fabrication methods in RF MEMS switches. J. Electron. Mater. 2021, 50, 3149–3168. [Google Scholar]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.; Khotkevich, V.; Morozov, S.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ali, M.B.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of l-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Sun, T.; Song, X.; Ran, Q.; Yu, C.; Yang, J.; Feng, H.; Yu, L.; Wei, D. Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology 2017, 28, 315501. [Google Scholar] [CrossRef]
- Kauling, A.P.; Seefeldt, A.T.; Pisoni, D.P.; Pradeep, R.C.; Bentini, R.; Oliveira, R.V.; Novoselov, K.S.; Castro Neto, A.H. The worldwide graphene flake production. Adv. Mater. 2018, 30, 1803784. [Google Scholar] [CrossRef] [PubMed]
- Allagui, A.; Abdelkareem, M.A.; Alawadhi, H.; Elwakil, A.S. Reduced graphene oxide thin film on conductive substrates by bipolar electrochemistry. Sci. Rep. 2016, 6, 21282. [Google Scholar] [CrossRef] [PubMed]
- Khakpour, I.; Rabiei Baboukani, A.; Allagui, A.; Wang, C. Bipolar exfoliation and in situ deposition of high-quality graphene for supercapacitor application. ACS Appl. Energy Mater. 2019, 2, 4813–4820. [Google Scholar] [CrossRef]
- Suter, J.L.; Sinclair, R.C.; Coveney, P.V. Principles governing control of aggregation and dispersion of graphene and graphene oxide in polymer melts. Adv. Mater. 2020, 32, 2003213. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Han, T.H.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Khakpour, I.; Baboukani, A.R.; Forouzanfar, S.; Allagui, A.; Wang, C. In-situ exfoliation and integration of vertically aligned graphene for high-frequency response on-chip microsupercapacitors. J. Power Sources 2021, 516, 230701. [Google Scholar] [CrossRef]
- Khakpour, I.; Baboukani, A.R.; Allagui, A.; Hachicha, A.A.; Wang, C. On the mechanistic pathways of exfoliation-and-deposition of graphene by bipolar electrochemistry. Nanotechnology 2021, 32, 345603. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q. Rev. Biophys. 1978, 11, 179–246. [Google Scholar] [CrossRef]
- Liu, X.; Shuai, H.-L.; Huang, K.-J. A label-free electrochemical aptasensor based on leaf-like vanadium disulfide-Au nanoparticles for the sensitive and selective detection of platelet-derived growth factor BB. Anal. Methods 2015, 7, 8277–8284. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Razmi, N.; Mokhtarzadeh, A.; Shadjou, N.; Mahboob, S. Aptamer based assay of plated-derived grow factor in unprocessed human plasma sample and MCF-7 breast cancer cell lysates using gold nanoparticle supported α-cyclodextrin. Int. J. Biol. Macromol. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, C.; Zhang, S.; He, L.; Wang, M.; Peng, D.; Tian, J.; Fang, S. Carbon-based nanocomposites with aptamer-templated silver nanoclusters for the highly sensitive and selective detection of platelet-derived growth factor. Biosens. Bioelectron. 2017, 89, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Leitzel, K.; Bryce, W.; Tomita, J.; Manderino, G.; Tribby, I.; Thomason, A.; Billingsley, M.; Podczaski, E.; Harvey, H.; Bartholomew, M. Elevated plasma platelet-derived growth factor B-chain levels in cancer patients. Cancer Res. 1991, 51, 4149–4154. [Google Scholar] [PubMed]
- Li, Y.; Liu, Z.; Lu, W.; Zhao, M.; Xiao, H.; Hu, T.; Ma, J.; Zheng, Z.; Jia, J.; Wu, H. Label-free electrochemical aptasensor based on the core-shell Cu-MOF@ TpBD hybrid nanoarchitecture for the sensitive detection of PDGF-BB. Analyst 2020, 146, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tian, D.; Zhang, L.; Guo, Q.; Cui, Y.; Yang, M. Dual signal amplification strategy for amperometric aptasensing using hydroxyapatite nanoparticles. Application to the sensitive detection of the cancer biomarker platelet-derived growth factor BB. Microchim. Acta 2017, 184, 4375–4381. [Google Scholar] [CrossRef]






| Electrode | Modification | Technique | Detection Strategy | LoD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| GCE | Cu-MOFs/TpBD-COFs | DPV | label-free | 0.034 pg mL−1 | 0.0001–60 ng mL−1 | [46] |
| HAP-NPs | SWV | labeled: HAP-NPs | 50 fg mL−1 | 0.1 pg mL−1–10 ng mL−1 | [47] | |
| C-MEMS thin film | oxygen–plasma etching | CV | label-free | 7 pM | 0.01–50 nM | [14] |
| EIS | 1.9 pM | 0.005–50 nM | ||||
| Au | AuNPs | SWV | labeled: α-cyclodextrin | 0.52 nM | 0.52–1.52 nM | [43] |
| graphene doped with silver nanoclusters | EIS | label-free | 26.5 fM | 32.3 fM–1.61 pM | [44] | |
| PET/Au | BPE-rGO | DPV | label-free | 0.65 pM | 0.0007–20 nM | [15] |
| C-MEMS | BPE-rGO | CV | label-free | 0.75 pM | 0.001–10 nM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forouzanfar, S.; Pala, N.; Wang, C. In-Situ Integration of 3D C-MEMS Microelectrodes with Bipolar Exfoliated Graphene for Label-Free Electrochemical Cancer Biomarkers Aptasensor. Micromachines 2022, 13, 104. https://doi.org/10.3390/mi13010104
Forouzanfar S, Pala N, Wang C. In-Situ Integration of 3D C-MEMS Microelectrodes with Bipolar Exfoliated Graphene for Label-Free Electrochemical Cancer Biomarkers Aptasensor. Micromachines. 2022; 13(1):104. https://doi.org/10.3390/mi13010104
Chicago/Turabian StyleForouzanfar, Shahrzad, Nezih Pala, and Chunlei Wang. 2022. "In-Situ Integration of 3D C-MEMS Microelectrodes with Bipolar Exfoliated Graphene for Label-Free Electrochemical Cancer Biomarkers Aptasensor" Micromachines 13, no. 1: 104. https://doi.org/10.3390/mi13010104
APA StyleForouzanfar, S., Pala, N., & Wang, C. (2022). In-Situ Integration of 3D C-MEMS Microelectrodes with Bipolar Exfoliated Graphene for Label-Free Electrochemical Cancer Biomarkers Aptasensor. Micromachines, 13(1), 104. https://doi.org/10.3390/mi13010104