Evaluation of Antifungal Properties of Titania P25
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.2. Characterization of Photocatalysts
2.3. The Photocatalytic Activity for Hydrogen Evolution under UV/vis Irradiation
2.4. Anifungal Tests
2.4.1. Mycelial Growth Test
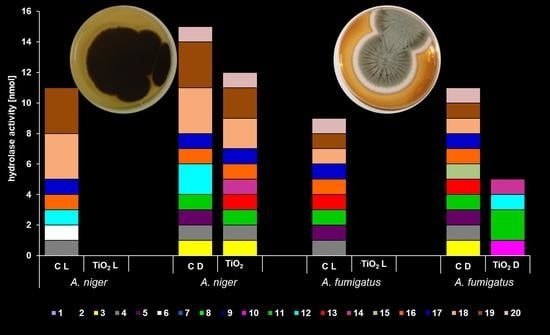
2.4.2. Enzymatic Activity
3. Results
3.1. Characterization of Photocatalysts
3.2. Hydrogen Evolution under UV/vis Irradiation
3.3. Anifungal Properties
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Martin, J.; Kraakman, N.J.R.; Perez, C.; Lebrero, R.; Munoz, R. A state-of-the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor Environmental Exposures and Exacerbation of Asthma: An Update to the 2000 Review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Markowska-Szczupak, A.; Wang, K.L.; Rokicka, P.; Endo, M.; Wei, Z.S.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photoch. Photobio. B 2015, 151, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Markowska-Szczupak, A.; Janda, K.; Wang, K.L.; Morawski, A.W.; Kowalska, E. Effect of Water Activity and Titania P25 Photocatalyst on Inactivation of Pathogenic Fungi—Contribution to the Protection of Public Health. Cent. Eur. J. Publ. Health 2015, 23, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Salas, B.; McCullagh, I.; Cranfield, K.; Fagan, C.; Geering, A.; Robb, A. COVID-19-Associated Pulmonary Aspergillosis: A Year-Long Retrospective Case Series. COVID 2022, 2, 976–982. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensive Care Med. 2021, 1, 71–80. [Google Scholar] [CrossRef]
- Pratiwi, C.; Rahayu, W.; Lioe, H.; Herawati, D.; Broto, W.; Ambarwati, S. The effect of temperature and relative humidity for Aspergillus flavus BIO 2237 growth and aflatoxin production on soybeans. Int. Food Res. J. 2015, 22, 82–87. [Google Scholar]
- Lal, P.M.; Arif, A.; Mohan, A.; Rackimuthu, S.; Hasan, M.M.; Islam, Z.; Uday, U.; Wara, U.U.; Shaikh, M.T.A.; Essar, M.Y. COVID-19 associated pulmonary aspergillosis (CAPA): An added potential burden on India’s pre-existing fungal superinfection. Clin. Epidemiol. Glob. Health 2022, 13, 100960. [Google Scholar] [CrossRef] [PubMed]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef]
- Burks, C.; Darby, A.; Gómez Londoño, L.; Momany, M.; Brewer, M.T. Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog. 2021, 17, e1009711. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B. Revisiting the fundamental physical chemistry in heterogeneous photocatalysis: Its thermodynamics and kinetics. Phys. Chem. Chem. Phys. 2014, 16, 1788–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, J.-M.; Disdier, J.; Pichat, P.; Malato, S.; Blanco, J. TiO2-based solar photocatalytic detoxification of water containing organic pollutants. Case studies of 2,4-dichlorophenoxyaceticacid (2,4-D) and of benzofuran. Appl. Catal. B Environ. 1998, 17, 15–23. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Rec. Patent. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide photocatalysts active in visible light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef]
- Mitoraj, D.; Janczyk, A.; Strus, M.; Kisch, H.; Stochel, G.; Heczko, P.B.; Macyk, W. Visible light inactivation of bacteria and fungi by modified titanium dioxide. Photochem. Photobiol. Sci. 2007, 6, 642–648. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core–shell photocatalysts. J. Mat. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Wang, K.L.; Janczarek, M.; Wei, Z.S.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photoch. Photobio. C 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Pichat, P.; Mozzanega, M.N.; Disdier, J.; Herrmann, J.M. Platinum content and temperature effects on the photocatalytic hydrogen production from aliphatic alcohols over platinum/titanium dioxide. Nouv. J. Chim. 1982, 6, 559–564. [Google Scholar]
- Ohtani, B.; Osaki, H.; Nishimoto, S.; Kagiya, T. A novel photocatalytic process of amine N-alkylation by platinized semiconductor particles suspended in alcohols. J. Am. Chem. Soc. 1986, 108, 308–310. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-governed performance of plasmonic photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Klein, M.; Hupka, J. Enhanced visible light photocatalytic activity of Pt/I-TiO2 in a slurry system and supported on glass packing. Sep. Purif. Technol. 2017, 189, 246–252. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Wei, Z.S.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-controlled synthesis of Pt particles on TiO2 surface: Physicochemical characteristic and photocatalytic activity. Catalysts 2019, 9, 940. [Google Scholar] [CrossRef] [Green Version]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Markowska-Szczupak, A.; Ulfig, K.; Grzmil, B.; Morawski, A.W. A preliminary study on antifungal effect of TiO2-based paints in natural indoor light. Pol. J. Chem. Technol. 2010, 12, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Thabet, S.; Simonet, F.; Lemaire, M.; Guillard, C.; Cotton, P. Impact of photocatalysis on fungal cells: Depiction of cellular and molecular effects on saccharomyces cerevisiae. Appl. Environ. Microb. 2014, 80, 7527–7535. [Google Scholar] [CrossRef] [Green Version]
- Thabet, S.; Weiss-Gayet, M.; Dappozze, F.; Cotton, P.; Guillard, C. Photocatalysis on yeast cells: Toward targets and mechanisms. Appl. Catal. B Environ. 2013, 140, 169–178. [Google Scholar] [CrossRef]
- Markov, S.L.; Vidakovic, A.M. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period. Technol. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, C.; Yoon, J. Developing a Testing Method for Antimicrobial Efficacy on TiO2 Photocatalytic Products. Environ. Eng. Res. 2008, 13, 136–140. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Colbeau-Justin, C.; Nitta, A.; Kowalska, E. P25 and its components—Electronic properties and photocatalytic activities. Surf. Interfaces 2022, 31, 102057. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photoch. Photobiol. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Mahaney, O.O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between photocatalytic activities and structural and physical properties of titanium(IV) oxide powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P 25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Lisowski, W.; Jarek, M.; Nowaczyk, G.; Zielińska-Jurek, A.; Zaleska, A. Visible light photoactivity of TiO2 loaded with monometallic (Au or Pt) and bimetallic (Au/Pt) nanoparticles. Appl. Surf. Sci. 2014, 317, 1131–1142. [Google Scholar] [CrossRef]
- Benz, D.; Felter, K.M.; Köser, J.; Thöming, J.; Mul, G.; Grozema, F.C.; Hintzen, H.T.; Kreutzer, M.T.; van Ommen, J.R. Assessing the Role of Pt Clusters on TiO2 (P25) on the Photocatalytic Degradation of Acid Blue 9 and Rhodamine B. J. Phys. Chem. C 2020, 124, 8269–8278. [Google Scholar] [CrossRef] [Green Version]
- Bielan, Z.; Sulowska, A.; Dudziak, S.; Siuzdak, K.; Ryl, J.; Zielinska-Jurek, A. Defective TiO2 core-shell magnetic photocatalyst modified with plasmonic nanoparticles for visible light-induced photocatalytic activity. Catalysts 2020, 10, 672. [Google Scholar] [CrossRef]
- Driessen, M.D.; Grassian, V.H. Photooxidation of Trichloroethylene on Pt/TiO2. J. Phys. Chem. B 1998, 102, 1418–1423. [Google Scholar] [CrossRef]
- Paszkiewicz, O.; Wang, K.; Rakoczy, R.; Kordas, M.; Leniec, G.; Kowalska, E.; Markowska-Szczupak, A. Antimicrobial properties of pristine and Pt-modified titania P25 in rotating magnetic field conditions. Chem. Eng. Process. Process Intensif. 2022, 178, 109010. [Google Scholar] [CrossRef]
- Wang, K.; Kowalska, E. Property-governed performance of platinum-modified titania photocatalysts. Front. Chem. 2022, 10, 972494. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.; Wendt, S.; Huo, P.; Hansen, J.Ø.; Streber, R.; Porsgaard, S.; Wei, Y.; Bechstein, R.; Lægsgaard, E.; Besenbacher, F. The Importance of Bulk Ti3+ Defects in the Oxygen Chemistry on Titania Surfaces. J. Am. Chem. Soc. 2011, 133, 6529–6532. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef]
- Baba, K.; Bulou, S.; Quesada-Gonzalez, M.; Bonot, S.; Collard, D.; Boscher, N.D.; Choquet, P. Significance of a noble metal nanolayer on the UV and visible light photocatalytic activity of anatase TiO2 thin films grown from a scalable PECVD/PVD approach. ACS Appl. Mater. Inter. 2017, 9, 41200–41209. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhang, C.; He, H. Formaldehyde Oxidation on Pd/TiO2 Catalysts at Room Temperature: The Effects of Surface Oxygen Vacancies. Top. Catal. 2020, 63, 810–816. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Vaiano, V.; Ciambelli, P.; Sannino, D. Photocatalytic Ethanol Oxidative Dehydrogenation over Pt/TiO2: Effect of the Addition of Blue Phosphors. Int. J. Photoenergy 2012, 2012, 687262. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Lu, Z.; Zhang, Y.; Su, Q.; Li, L. Hydrogen-Etched TiO2−x as Efficient Support of Gold Catalysts for Water–Gas Shift Reaction. Catalysts 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Abdelouahab Reddam, H.; Elmail, R.; Lloria, S.C.; Monrós Tomás, G.; Reddam, Z.A.; Coloma-Pascual, F. Synthesis of Fe, Mn and Cu modified TiO2 photocatalysts for photodegradation of Orange II. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 138–148. [Google Scholar] [CrossRef]
- Shu, Z.; Cai, Y.; Ji, J.; Tang, C.; Yu, S.; Zou, W.; Dong, L. Pt Deposites on TiO2 for Photocatalytic H2 Evolution: Pt Is Not Only the Cocatalyst, but Also the Defect Repair Agent. Catalysts 2020, 10, 1047. [Google Scholar] [CrossRef]
- Siuzdak, K.; Sawczak, M.; Klein, M.; Nowaczyk, G.; Jurga, S.; Cenian, A. Preparation of platinum modified titanium dioxide nanoparticles with the use of laser ablation in water. Phys. Chem. Chem. Phys. 2014, 16, 15199–15206. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qi, H.; Zhou, J.; Xu, W.; Niu, Y.; Zhang, B.; Zhao, Y.; Liu, W.; Ao, Z.; Kuang, Z.; et al. Encapsulation of Platinum by Titania under an Oxidative Atmosphere: Contrary to Classical Strong Metal–Support Interactions. ACS. Catal. 2021, 11, 6081–6090. [Google Scholar] [CrossRef]
- Endo, M.; Wei, Z.S.; Wang, K.L.; Karabiyik, B.; Yoshiiri, K.; Rokicka, P.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Noble metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotech. 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Viegas, C.; Viegas, S.; Brandão, J.; Alves-Correia, M.; Borrego, L.-M.; Clemons, K.V.; Stevens, D.A.; Richardson, M. The role of occupational Aspergillus exposure in the development of diseases. Med. Mycol. 2019, 57, S196–S205. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hameed, A.A.; Ayesh, A.M.; Abdel Razik Mohamed, M.; Abdel Mawla, H.F. Fungi and some mycotoxins producing species in the air of soybean and cotton mills: A case study. Atmos. Pollut. Res. 2012, 3, 126–131. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Saphira Radin Mohamed, R.M.; Talip, B.; Othman, N.; Hossain, S.; Vo, D.-V.N.; Alduais, N. Inactivation of fungal spores from clinical environment by silver bio-nanoparticles; optimization, artificial neural network model and mechanism. Environ. Res. 2022, 204, 111926. [Google Scholar] [CrossRef]
- Godlewska-Żyłkiewicz, B.; Sawicka, S.; Karpińska, J. Removal of Platinum and Palladium from Wastewater by Means of Biosorption on Fungi Aspergillus sp. and Yeast Saccharomyces sp. Water 2019, 11, 1522. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Acharya, K.; Biswas, A.; Jana, N.R. TiO2 Nanoparticles Co-doped with Nitrogen and Fluorine as Visible-Light-Activated Antifungal Agents. ACS Appl. Nano Mater. 2020, 3, 2016–2025. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Endo, M.; Wang, K.; Bahena, D.; Rodriguez-Lopez, J.L.; Remita, H. Inhibition of fungal growth using modified TiO2 with core@shell structure of Ag@CuO clusters. ACS Appl. Bio Mater. 2019, 2, 5626–5633. [Google Scholar] [CrossRef]
- Krishnan, A.; Convey, P.; Gonzalez-Rocha, G.; Alias, S.A. Production of extracellular hydrolase enzymes by fungi from King George Island. Polar Biol. 2016, 39, 65–76. [Google Scholar] [CrossRef]
- Calado, T.; Venâncio, A.; Abrunhosa, L. Irradiation for Mold and Mycotoxin Control: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef]
- Yagyu, Y.; Sakudo, A. Current technology and applications of gas plasma for disinfection of agricultural products: Disinfection of fungal spores on Citrus unshiu by atmospheric pressure dielectric barrier discharge. In Gas Plasma Sterilization in Microbiology: Theory, Applications, Pitfalls and New Perspectives; Shintani, H., Sakudo, A., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 116–120. [Google Scholar]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Rogawansamy, S.; Gaskin, S.; Taylor, M.; Pisaniello, D. An Evaluation of Antifungal Agents for the Treatment of Fungal Contamination in Indoor Air Environments. Int. J. Env. Res. Pub. Health 2015, 12, 6319–6332. [Google Scholar] [CrossRef] [Green Version]
- Hojnik, N.; Modic, M.; Ni, Y.; Filipič, G.; Cvelbar, U.; Walsh, J.L. Effective Fungal Spore Inactivation with an Environmentally Friendly Approach Based on Atmospheric Pressure Air Plasma. Environ. Sci. Technol. 2019, 53, 1893–1904. [Google Scholar] [CrossRef]
- Escudero-Leyva, E.; Alfaro-Vargas, P.; Muñoz-Arrieta, R.; Charpentier-Alfaro, C.; Granados-Montero, M.d.M.; Valverde-Madrigal, K.S.; Pérez-Villanueva, M.; Méndez-Rivera, M.; Rodríguez-Rodríguez, C.E.; Chaverri, P.; et al. Tolerance and Biological Removal of Fungicides by Trichoderma Species Isolated From the Endosphere of Wild Rubiaceae Plants. Front. Agron. 2022, 3, 772170. [Google Scholar] [CrossRef]







| No | Enzyme | Substrate |
|---|---|---|
| 1. | Control | |
| 2. | Alkaline phosphatase | 2-naphtyl phosphate |
| 3. | Esterase (C4) | 2-naphtyl butyrate |
| 4. | Esterase Lipase (C8) | 2-naphtyl caprylate |
| 5. | Lipase (C14) | 2-naphtyl myristate |
| 6. | Leucine arylamidase | L-leucyl-2-naphthylamide |
| 7. | Valine arylamidase | L-valyl-2-naphthylamide |
| 8. | Cystine arylamidase | L-valyl-2-naphthylamide |
| 9. | Trypsin | N-benzoyl-DL-arginine-2-naphtylamide |
| 10. | α-chymotrypsin | N-glutatyl-phenylalanine-2-naphthylamide |
| 11. | Acid phosphatase | 2-naphthyl phosphate |
| 12. | Naphthol-AS-BI-phosphohydrolase | Naphthol-AS-BI-phosphate |
| 13. | α-galactosidase | 6-Br-2-naphthyl-αD-galactopyranoside |
| 14. | β-galactosidase | 2-naphthyl-βD-galactopyranoside |
| 15. | β-glucuronidase | Naphthol-AS-BI-βD-glucuronide |
| 16. | α-glucosidase | 2-naphthyl-αD-glucopyranoside |
| 17. | β-glucosidase | 6-Br-2-naphthyl- βD-glucopyranoside |
| 18. | N-acetyl-β-glucosaminidase | 1-naphthyl-N-acetyl-βD-glucosamide |
| 19. | α-mannosidase | 6-Br-2-naphthyl- αD-mannopyranoside |
| 20. | α-fucosidase | 2-naphthyl-αL-fucopyranoside |
| Sample Name | Crystalline Composition (%) | Particle Size (nm) | ||
|---|---|---|---|---|
| Anatase | Rutile | NC | ||
| HomoP25 | 77.0 | 13.8 | 9.2 | 119.1 |
| HomoP25-200 | 74.8 | 13.6 | 11.6 | 141.6 |
| HomoP25-300 | 71.2 | 13.4 | 15.4 | 187.8 |
| HomoP25-500 | 69.3 | 14.4 | 16.3 | 521.9 |
| Sample Name | Oxygen (1 s) | Titanium (2p3/2) | O/Ti Molar Ratio | |||
|---|---|---|---|---|---|---|
| O-H | Ti-OH/C=O | TiO2 | Ti4+ | Ti3+ | ||
| HomoP25 | 8.6 | 34.6 | 56.8 | 96.4 | 3.6 | 2.6 |
| HomoP25-200 | 14.1 | 33.1 | 52.8 | 93.4 | 6.6 | 2.9 |
| HomoP25-300 | 10.4 | 37.8 | 51.8 | 95.8 | 4.2 | 2.9 |
| HomoP25-500 | 2.1 | 51.7 | 46.2 | 95.1 | 4.9 | 3.7 |
| Photocatalysts Name | Negative Control | HomoP25 | HomoP25-200 | HomoP25-300 | HomoP25-500 | 0.5Pt-HomoP25 | 2.0Pt-HomoP25 | Negative Control | HomoP25 | HomoP25-200 | HomoP25-300 | HomoP25-500 | 0.5Pt-HomoP25 | 2.0Pt-HomoP25 | Negative Control | HomoP25 | HomoP25-200 | HomoP25-300 | HomoP25-500 | 0.5Pt-HomoP25 | 2.0Pt-HomoP25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| irradiation [min] | 60 | 120 | 180 | ||||||||||||||||||
 A. niger [logCFU/mL] | 7.6 | 7.1 | 7.0 | 0.0 | 0.0 | 7.3 | 7.2 | 7.6 | 6.9 | 6.7 | 0.0 | 0.0 | 7.1 | 7.1 | 7.5 | 6.6 | 6.5 | 0.0 | 0.0 | 6.8 | 6.4 |
 A. fumigatus [logCFU/mL] | 7.6 | 7.6 | 6.8 | 5.1 | 0.0 | 7.4 | 6.2 | 7.6 | 7.0 | 6.2 | 0.0 | 0.0 | 7.2 | 6.1 | 7.6 | 7.0 | 6.1 | 0.0 | 0.0 | 6.9 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Paszkiewicz, O.; Vincent, M.; Henkiel, P.; Kowalski, D.; Kowalska, E.; Markowska-Szczupak, A. Evaluation of Antifungal Properties of Titania P25. Micromachines 2022, 13, 1851. https://doi.org/10.3390/mi13111851
Wang K, Paszkiewicz O, Vincent M, Henkiel P, Kowalski D, Kowalska E, Markowska-Szczupak A. Evaluation of Antifungal Properties of Titania P25. Micromachines. 2022; 13(11):1851. https://doi.org/10.3390/mi13111851
Chicago/Turabian StyleWang, Kunlei, Oliwia Paszkiewicz, Mewin Vincent, Patrycja Henkiel, Damian Kowalski, Ewa Kowalska, and Agata Markowska-Szczupak. 2022. "Evaluation of Antifungal Properties of Titania P25" Micromachines 13, no. 11: 1851. https://doi.org/10.3390/mi13111851
APA StyleWang, K., Paszkiewicz, O., Vincent, M., Henkiel, P., Kowalski, D., Kowalska, E., & Markowska-Szczupak, A. (2022). Evaluation of Antifungal Properties of Titania P25. Micromachines, 13(11), 1851. https://doi.org/10.3390/mi13111851