Recent Advances in Thermoplastic Microfluidic Bonding
Abstract
:1. Introduction
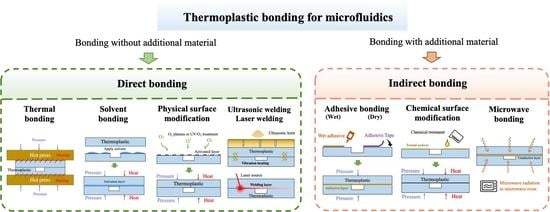
Thermoplastic Bonding Strategies in Microfluidics
2. Direct Bonding
2.1. Thermal Fusion Bonding
2.2. Solvent Bonding
2.3. Physical Surface Modification
2.4. Ultrasonic and Laser Welding
3. Indirect Bonding
3.1. Adhesive Bonding
3.2. Chemical Surface Modification
3.3. Microwave Bonding
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jerald, J.; Asokan, P.; Prabakaran, R. A Comprehensive Review on Microfluidics Technology and its Applications. In Recent Advances in Mechanical Engineering; Springer: Singapore, 2020; pp. 235–245. [Google Scholar]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Araci, I.E.; Quake, S.R. Microfluidic very large scale integration (mVLSI) with integrated micromechanical valves. Lab Chip 2012, 12, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Mehling, M.; Tay, S. Microfluidic cell culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. OnLine 2020, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. Engl. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Tsao, C.W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Material Properties of Polystyrene and Poly(methyl methacrylate) (PMMA) Microspheres. Available online: https://www.bangslabs.com/material-properties-polystyrene-and-polymethyl-methacrylate-pmma-microspheres (accessed on 25 February 2022).
- Roth, B.; Drummer, D. Pressure Equilibrium Time of a Cyclic-Olefin Copolymer. Polymer 2021, 13, 2309. [Google Scholar] [CrossRef] [PubMed]
- Direct Plastics Ltd. Polycarbonate Material Data Sheet. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiFj5Hoq9L2AhUGGKYKHS-GCvYQFnoECAIQAQ&url=https%3A%2F%2Fwww.directplastics.co.uk%2Fpdf%2Fdatasheets%2FPolycarbonate%2520Data%2520Sheet.pdf&usg=AOvVaw0cNaiR-1MqGjrElN-2BVXX (accessed on 25 February 2022).
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.; Beebe, D.J.; Young, E.W. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Suriano, R.; Kuznetsov, A.; Eaton, S.M.; Kiyan, R.; Cerullo, G.; Osellame, R.; Chichkov, B.N.; Levi, M.; Turri, S. Femtosecond laser ablation of polymeric substrates for the fabrication of microfluidic channels. Appl. Surf. Sci. 2011, 257, 6243–6250. [Google Scholar] [CrossRef]
- Liu, K.; Xiang, J.; Ai, Z.; Zhang, S.; Fang, Y.; Chen, T.; Zhou, Q.; Li, S.; Wang, S.; Zhang, N. PMMA microfluidic chip fabrication using laser ablation and low temperature bonding with OCA film and LOCA. Microsyst. Technol. 2016, 23, 1937–1942. [Google Scholar] [CrossRef]
- Ho, C.M.; Ng, S.H.; Li, K.H.; Yoon, Y.J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Abgrall, P.; Low, L.N.; Nguyen, N.T. Fabrication of planar nanofluidic channels in a thermoplastic by hot-embossing and thermal bonding. Lab Chip 2007, 7, 520–522. [Google Scholar] [CrossRef]
- Focke, M.; Kosse, D.; Muller, C.; Reinecke, H.; Zengerle, R.; von Stetten, F. Lab-on-a-Foil: Microfluidics on thin and flexible films. Lab Chip 2010, 10, 1365–1386. [Google Scholar] [CrossRef]
- Velten, T.; Schuck, H.; Richter, M.; Klink, G.; Bock, K.; Malek, C.K.; Roth, S.; Scho, H.; Bolt, P.J. Microfluidics on foil: State of the art and new developments. Proc. Inst. Mech. Eng. Part B-J. Eng. Manuf. 2008, 222, 107–116. [Google Scholar] [CrossRef]
- Mair, D.A.; Geiger, E.; Pisano, A.P.; Frechet, J.M.; Svec, F. Injection molded microfluidic chips featuring integrated interconnects. Lab Chip 2006, 6, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluid. 2009, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Giselbrecht, S.; Gietzelt, T.; Gottwald, E.; Trautmann, C.; Truckenmuller, R.; Weibezahn, K.F.; Welle, A. 3D tissue culture substrates produced by microthermoforming of pre-processed polymer films. Biomed. Microdev. 2006, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Gartner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Temiz, Y.; Lovchik, R.D.; Kaigala, G.V.; Delamarche, E. Lab-on-a-chip devices: How to close and plug the lab? Microelectron. Eng. 2015, 132, 156–175. [Google Scholar] [CrossRef]
- Cao, Y.; Bontrager-Singer, J.; Zhu, L. A 3D microfluidic device fabrication method using thermopress bonding with multiple layers of polystyrene film. J. Micromech. Microeng. 2015, 25, 65005. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, L.; Chen, G. A spring-driven press device for hot embossing and thermal bonding of PMMA microfluidic chips. Electrophoresis 2010, 31, 2512–2519. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.Y.; Chen, G. Hot embossing and thermal bonding of poly(methyl methacrylate) microfluidic chips using positive temperature coefficient ceramic heater. Anal. Bioanal. Chem. 2011, 401, 2657–2665. [Google Scholar] [CrossRef]
- Yu, S.Z.; Ng, S.P.; Wang, Z.F.; Tham, C.L.; Soh, Y.C. Thermal bonding of thermoplastic elastomer film to PMMA for microfluidic applications. Surf. Coat. Technol. 2017, 320, 437–440. [Google Scholar] [CrossRef]
- Gleichweit, E.; Baumgartner, C.; Diethardt, R.; Murer, A.; Sallegger, W.; Werkl, D.; Köstler, S. UV/Ozone Surface Treatment for Bonding of Elastomeric COC-Based Microfluidic Devices. In Multidisciplinary Digital Publishing Institute Proceedings; MDPI: Basel, Switzerland, 2018; Volume 2. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Yue, C.Y.; Lam, Y.C.; Roy, S.; Jena, R.K. A Modified Quasi-Creep Model for Assessment of Deformation of Topas COC Substrates in the Thermal Bonding of Microfluidic Devices: Experiments and Modeling. J. Appl. Polym. Sci. 2011, 122, 867–873. [Google Scholar] [CrossRef]
- Kurihara, K.; Hokari, R.; Satoh, T.; Sugiura, S.; Miyake, K.; Kanamori, T. Low-deformation precision thermal bonding of nanostructured microfluidic chips. Jpn. J. Appl. Phys. 2020, 59, SIIJ08. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Lin, J.; Su, R.; Xie, Y. Vacuum-assisted thermal bonding of plastic capillary electrophoresis microchip imprinted with stainless steel template. J. Chromatogr. A 2004, 1038, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.R.; Sun, P.K.; Mayer, M.; Besser, R.S. Gas-assisted thermal bonding of thermoplastics for the fabrication of microfluidic devices. Microsyst. Technol. 2019, 25, 3923–3932. [Google Scholar] [CrossRef]
- Park, T.; Song, I.H.; Park, D.S.; You, B.H.; Murphy, M.C. Thermoplastic fusion bonding using a pressure-assisted boiling point control system. Lab Chip 2012, 12, 2799–2802. [Google Scholar] [CrossRef] [PubMed]
- Mekaru, H. Thermal bonding of polyimide to form sealed microchannels. Jpn. J. Appl. Phys. 2017, 56, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.L.; Liu, G.; Guo, Y.H.; Tian, Y.C. Study of PMMA thermal bonding. Microsyst. Technol. 2007, 13, 403–407. [Google Scholar] [CrossRef]
- Studer, V.; Pepin, A.; Chen, Y. Nanoembossing of thermoplastic polymers for microfluidic applications. Appl. Phys. Lett. 2002, 80, 3614–3616. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Chang, M.-C.; Shih, Z.-F.; Lien, Y.-H.; Tsao, C.-W. Cyclic Block Copolymer Microchannel Fabrication and Sealing for Microfluidics Applications. Inventions 2018, 3, 49. [Google Scholar] [CrossRef] [Green Version]
- Busek, M.; Novik, S.; Aizenshtadt, A.; Amirola-Martinez, M.; Combriat, T.; Grunzner, S.; Krauss, S. Thermoplastic Elastomer (TPE)-Poly(Methyl Methacrylate) (PMMA) Hybrid Devices for Active Pumping PDMS-Free Organ-on-a-Chip Systems. Biosensors 2021, 11, 162. [Google Scholar] [CrossRef]
- Yin, Z.F.; Qi, L.P.; Zou, H.L.; Sun, L.; Xu, S.B. A novel bonding method for fabrication of PET planar nanofluidic chip with low dimension loss and high bonding strength. J. Micromech. Microeng. 2015, 25, 85015. [Google Scholar] [CrossRef]
- Uba, F.I.; Hu, B.; Weerakoon-Ratnayake, K.; Oliver-Calixte, N.; Soper, S.A. High process yield rates of thermoplastic nanofluidic devices using a hybrid thermal assembly technique. Lab Chip 2015, 15, 1038–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkubo, Y.; Endo, K.; Yamamura, K. Adhesive-free adhesion between heat-assisted plasma-treated fluoropolymers (PTFE, PFA) and plasma-jet-treated polydimethylsiloxane (PDMS) and its application. Sci. Rep. 2018, 8, 18058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uba, F.I.; Pullagurla, S.R.; Sirasunthorn, N.; Wu, J.; Park, S.; Chantiwas, R.; Cho, Y.K.; Shin, H.; Soper, S.A. Surface charge, electroosmotic flow and DNA extension in chemically modified thermoplastic nanoslits and nanochannels. Analyst 2015, 140, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Du, L.Q.; Chang, H.L.; Song, M.C.; Liu, C. A method of water pretreatment to improve the thermal bonding rate of PMMA microfluidic chip. Microsyst. Technol. 2012, 18, 423–428. [Google Scholar] [CrossRef]
- Gong, Y.; Park, J.M.; Lim, J. An Interference-Assisted Thermal Bonding Method for the Fabrication of Thermoplastic Microfluidic Devices. Micromachines 2016, 7, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, M.J.T.; Nieuwoudt, M.; Yong, R.M.; Vanholsbeeck, F.; Williams, D.E.; Simpson, M.C. Excellent quality microchannels for rapid microdevice prototyping: Direct CO2 laser writing with efficient chemical postprocessing. Microfluid. Nanofluid. 2019, 23, 1–13. [Google Scholar] [CrossRef]
- Cassano, C.L.; Simon, A.J.; Liu, W.; Fredrickson, C.; Fan, Z.H. Use of vacuum bagging for fabricating thermoplastic microfluidic devices. Lab Chip 2015, 15, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Trinh, K.T.L.; Thai, D.A.; Chae, W.R.; Lee, N.Y. Rapid Fabrication of Poly(methyl methacrylate) Devices for Lab-on-a-Chip Applications Using Acetic Acid and UV Treatment. Acs Omega 2020, 5, 17396–17404. [Google Scholar] [CrossRef]
- Faghih, M.M.; Sharp, M.K. Solvent-based bonding of PMMA–PMMA for microfluidic applications. Microsyst. Technol. 2018, 25, 3547–3558. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.W.; Li, T.; Han, X.W. Miscible Organic Solvents Soak Bonding Method Use in a PMMA Multilayer Microfluidic Device. Micromachines 2014, 5, 1416–1428. [Google Scholar] [CrossRef] [Green Version]
- Wan, A.M.; Moore, T.A.; Young, E.W. Solvent Bonding for Fabrication of PMMA and COP Microfluidic Devices. J. Vis. Exp. 2017, 119, e55175. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Takahashi, S.; Hatakeyama, K.; Kamei, K.I. Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures. Micromachines 2021, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Wiria, F.E.; Tay, N.B. Low Distortion Solvent Bonding of Microfluidic Chips. Procedia Eng. 2016, 141, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.H.; Tjeung, R.T.; Wang, Z.F.; Lu, A.C.W.; Rodriguez, I.; de Rooij, N.F. Thermally activated solvent bonding of polymers. Microsyst. Technol. 2008, 14, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.F.; Wang, W.H. A novel bonding method for fabrication of PMMA nanofluidic chip with low deformation of the nano-trenches. Microfluid. Nanofluid. 2018, 22, 99. [Google Scholar] [CrossRef]
- Su, S.S.; Jing, G.S.; Zhang, M.Q.; Liu, B.X.; Zhu, X.R.; Wang, B.; Fu, M.Z.; Zhu, L.X.; Cheng, J.; Guo, Y. One-step bonding and hydrophobic surface modification method for rapid fabrication of polycarbonate-based droplet microfluidic chips. Sens. Actuators B Chem. 2019, 282, 60–68. [Google Scholar] [CrossRef]
- Keller, N.; Nargang, T.M.; Runck, M.; Kotz, F.; Striegel, A.; Sachsenheimer, K.; Klemm, D.; Lange, K.; Worgull, M.; Richter, C.; et al. Tacky cyclic olefin copolymer: A biocompatible bonding technique for the fabrication of microfluidic channels in COC. Lab Chip 2016, 16, 1561–1564. [Google Scholar] [CrossRef] [Green Version]
- Gan, Z.B.; Zhang, L.Y.; Chen, G. Solvent bonding of poly(methyl methacrylate) microfluidic chip using phase-changing agar hydrogel as a sacrificial layer. Electrophoresis 2011, 32, 3319–3323. [Google Scholar] [CrossRef]
- Rahbar, M.; Chhina, S.; Sameoto, D.; Parameswaran, M. Microwave-induced, thermally assisted solvent bonding for low-cost PMMA microfluidic devices. J. Micromech. Microeng. 2010, 20, 15026. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications. Biosensors 2021, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.; Sadri, A.; Young, E.W. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab Chip 2015, 15, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Bamshad, A.; Nikfarjam, A.; Khaleghi, H. A new simple and fast thermally-solvent assisted method to bond PMMA-PMMA in micro-fluidics devices. J. Micromech. Microeng. 2016, 26, 65017. [Google Scholar] [CrossRef]
- Lynh, H.D.; Pin-Chuan, C. Novel solvent bonding method for creation of a three-dimensional, non-planar, hybrid PLA/PMMA microfluidic chip. Sens. Actuators A Phys. 2018, 280, 350–358. [Google Scholar] [CrossRef]
- Chen, P.C.; Duong, L.H. Novel solvent bonding method for thermoplastic microfluidic chips. Sens. Actuators B Chem. 2016, 237, 556–562. [Google Scholar] [CrossRef]
- Chen, P.C.; Lin, Y.T.; Truong, C.M.; Chen, P.S.; Chiang, H.K. Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips. Sensors 2021, 21, 698. [Google Scholar] [CrossRef]
- Zoupanou, S.; Chiriaco, M.S.; Tarantini, I.; Ferrara, F. Innovative 3D Microfluidic Tools for On-Chip Fluids and Particles Manipulation: From Design to Experimental Validation. Micromachines 2021, 12, 104. [Google Scholar] [CrossRef]
- Duong, L.H.; Chen, P.C. Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 2019, 13, 24108. [Google Scholar] [CrossRef]
- Nemati, S.H.; Liyu, D.A.; Canul, A.J.; Vasdekis, A.E. Solvent immersion imprint lithography: A high-performance, semi-automated procedure. Biomicrofluidics 2017, 11, 24111. [Google Scholar] [CrossRef] [Green Version]
- Akhil, A.V.; Raj, D.D.D.; Raj, M.K.; Bhat, S.R.; Akshay, V.; Bhowmik, S.; Ramanathan, S.; Ahmed, S. Vaporized solvent bonding of polymethyl methacrylate. J. Adhes. Sci. Technol. 2016, 30, 826–841. [Google Scholar] [CrossRef]
- Wouters, S.; De Vos, J.; Dores-Sousa, J.L.; Wouters, B.; Desmet, G.; Eeltink, S. Prototyping of thermoplastic microfluidic chips and their application in high-performance liquid chromatography separations of small molecules. J. Chromatogr. A 2017, 1523, 224–233. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Horowitz, L.F.; Castro, K.; Kenerson, H.; Bhattacharjee, N.; Gandhe, G.; Raman, A.; Monnat, R.J.; Yeung, R.; Rostomily, R.C.; et al. A microfluidic platform for functional testing of cancer drugs on intact tumor slices. Lab Chip 2020, 20, 1658–1675. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Tweedie, M.; Gajula, D.R.; Ward, B.; Maguire, P.D. High-strength thermoplastic bonding for multi-channel, multi-layer lab-on-chip devices for ocean and environmental applications. Microfluid. Nanofluid. 2015, 19, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Bagiatis, V.; Critchlow, G.W.; Price, D.; Wang, S. The effect of atmospheric pressure plasma treatment (APPT) on the adhesive bonding of poly(methyl methacrylate) (PMMA)-to-glass using a polydimethylsiloxane (PDMS)-based adhesive. Int. J. Adhes. Adhes. 2019, 95, 102405. [Google Scholar] [CrossRef]
- Chiang, C.C.; Immanuel, P.N.; Chiu, Y.H.; Huang, S.J. Heterogeneous Bonding of PMMA and Double-Sided Polished Silicon Wafers through H2O Plasma Treatment for Microfluidic Devices. Coatings 2021, 11, 580. [Google Scholar] [CrossRef]
- Shinohara, H.; Mizuno, J.; Shoji, S. Studies on low-temperature direct bonding of VUV, VUV/O-3 and O-2 plasma pretreated cyclo-olefin polymer. Sens. Actuators A Phys. 2011, 165, 124–131. [Google Scholar] [CrossRef]
- Qu, X.T.; Li, J.L.; Yin, Z.F. A novel bonding method for large scale poly(methyl methacrylate) micro- and nanofluidic chip fabrication. Jpn. J. Appl. Phys. 2018, 57, 47001. [Google Scholar] [CrossRef]
- Vourdas, N.; Tserepi, A.; Boudouvis, A.G.; Gogolides, E. Plasma processing for polymeric microfluidics fabrication and surface modification: Effect of super-hydrophobic walls on electroosmotic flow. Microelectron. Eng 2008, 85, 1124–1127. [Google Scholar] [CrossRef]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Lee, N.Y. Solvent-free thermoplastic-poly(dimethylsiloxane) bonding mediated by UV irradiation followed by gas-phase chemical deposition of an adhesion linker. J. Micromech. Microeng. 2015, 25, 75007. [Google Scholar] [CrossRef]
- Song, K.Y.; Zhang, H.B.; Zhang, W.J.; Teixeira, A. Enhancement of the surface free energy of PDMS for reversible and leakage-free bonding of PDMS-PS microfluidic cell-culture systems. Microfluid. Nanofluid. 2018, 22, 1–9. [Google Scholar] [CrossRef]
- Immanuel, P.N.; Chiang, C.C.; Yang, C.R.; Subramani, M.; Lee, T.H.; Huang, S.J. Surface activation of poly(methyl methacrylate) for microfluidic device bonding through a H2O plasma treatment linked with a low-temperature annealing. J. Micromech. Microeng. 2021, 31, 55004. [Google Scholar] [CrossRef]
- Terai, H.; Funahashi, R.; Hashimoto, T.; Kakuta, M. Heterogeneous bonding between cyclo-olefin polymer (COP) and glass-like substrate by newly developed water vapor-assisted plasma, Aqua Plasma Cleaner. Electr. Eng. Jpn. 2018, 205, 48–56. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, C.; Qiao, H.C.; Zhu, L.Y.; Chen, G.; Dai, X.D. Hot embossing/bonding of a poly(ethylene terephthalate) (PET) microfluidic chip. J. Micromech. Microeng. 2008, 18, 15008. [Google Scholar] [CrossRef]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Lam, Y.C. Influence of plasma surface treatment on thermal bonding and flow behavior in Cyclic Olefin Copolymer (COC) based microfluidic devices. Vacuum 2011, 85, 1102–1104. [Google Scholar] [CrossRef]
- Liu, J.S.; Qiao, H.C.; Liu, C.; Xu, Z.; Li, Y.Q.; Wang, L.D. Plasma assisted thermal bonding for PMMA microfluidic chips with integrated metal microelectrodes. Sens. Actuators B Chem. 2009, 141, 646–651. [Google Scholar] [CrossRef]
- Tennico, Y.H.; Koesdjojo, M.T.; Kondo, S.; Mandrell, D.T.; Remcho, V.T. Surface modification-assisted bonding of polymer-based microfluidic devices. Sens. Actuators B Chem. 2010, 143, 799–804. [Google Scholar] [CrossRef]
- El Fissi, L.; Vandormael, D.; Francis, L.A. Direct assembly of cyclic olefin copolymer microfluidic devices helped by dry photoresist. Sens. Actuators A Phys. 2015, 223, 76–83. [Google Scholar] [CrossRef]
- Hassanpour-Tamrin, S.; Sanati-Nezhad, A.; Sen, A. A simple and low-cost approach for irreversible bonding of polymethylmethacrylate and polydimethylsiloxane at room temperature for high-pressure hybrid microfluidics. Sci. Rep. 2021, 11, 4821. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Li, H.W.; Yi, Y.; Foulds, I.G. PMMA to Polystyrene bonding for polymer based microfluidic systems. Microsyst. Technol. 2014, 20, 59–64. [Google Scholar] [CrossRef]
- Truckenmüller, R.; Cheng, Y.; Ahrens, R.; Bahrs, H.; Fischer, G.; Lehmann, J. Micro ultrasonic welding: Joining of chemically inert polymer microparts for single material fluidic components and systems. Microsyst. Technol. 2006, 12, 1027–1029. [Google Scholar] [CrossRef]
- Yu, H.; Tor, S.B.; Loh, N.H. Rapid bonding enhancement by auxiliary ultrasonic actuation for the fabrication of cyclic olefin copolymer (COC) microfluidic devices. J. Micromech. Microeng. 2014, 24, 115020. [Google Scholar] [CrossRef]
- Zongbo, Z.; Xiaodong, W.; Yi, L.; Zhenqiang, Z.; Liding, W. Study on Heating Process of Ultrasonic Welding for Thermoplastics. J. Thermoplast. Compos. Mater. 2009, 23, 647–664. [Google Scholar] [CrossRef]
- Jongbaeg, K.; Bongwon, J.; Mu, C.; Liwei, L. Ultrasonic Bonding for MEMS Sealing and Packaging. IEEE Trans. Adv. Packag. 2009, 32, 461–467. [Google Scholar] [CrossRef]
- Villegas, I.F. In situ monitoring of ultrasonic welding of thermoplastic composites through power and displacement data. J. Thermoplast. Compos. Mater. 2013, 28, 66–85. [Google Scholar] [CrossRef]
- Lee, K.G.; Shin, S.; Kim, B.I.; Bae, N.H.; Lee, M.K.; Lee, S.J.; Lee, T.J. Ultrasonic bonding method for heterogeneous microstructures using self-balancing jig. Lab Chip 2015, 15, 1412–1416. [Google Scholar] [CrossRef]
- Sun, Y.B.; Teng, T.D.; Guo, G.Q.; Wu, G.X. Ultrasonic bonding method controlled by the characteristic waveform of ultrasonic propagation. Micro Nano Lett. 2019, 14, 547–550. [Google Scholar] [CrossRef]
- Li, S.W.; Xu, J.H.; Wang, Y.J.; Lu, Y.C.; Luo, G.S. Low-temperature bonding of poly-(methyl methacrylate) microfluidic devices under an ultrasonic field. J. Micromech. Microeng. 2009, 19, 15035. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Luo, Y.; He, S.; Wang, L. Thermal assisted ultrasonic bonding method for poly(methyl methacrylate) (PMMA) microfluidic devices. Talanta 2010, 81, 1331–1338. [Google Scholar] [CrossRef]
- Kistrup, K.; Poulsen, C.E.; Hansen, M.F.; Wolff, A. Ultrasonic welding for fast bonding of self-aligned structures in lab-on-a-chip systems. Lab Chip 2015, 15, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.H.; Zhang, G.H.; Asao, M.; Zhang, M.; Feng, H.X.; Wu, Y.B. Study on the novel ultrasonic weld properties of heterogeneous polymers between PC and PMMA. Int. J. Adhes. Adhes. 2010, 30, 729–734. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Luo, Y.; Wang, X.D.; Zheng, Y.S.; Zhang, Y.G.; Wang, L.D. A low temperature ultrasonic bonding method for PMMA microfluidic chips. Microsyst. Technol. 2010, 16, 533–541. [Google Scholar] [CrossRef]
- Li, J.M.; Meng, F.J.; Liang, C.; Liu, C. Energy director structure and self-balancing jig for the ultrasonic bonding of microfluidic chips. Micro Nano Lett. 2017, 12, 453–457. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, Z.F.; de Rooij, N.F. Microfluidic connectors by ultrasonic welding. Microelectron. Eng. 2009, 86, 1354–1357. [Google Scholar] [CrossRef]
- Liang, C.; Liu, C.; Liu, Z.Y.; Meng, F.J.; Li, J.M. Laser-bulge based ultrasonic bonding method for fabricating multilayer thermoplastic microfluidic devices. J. Micromech. Microeng. 2017, 27, 115012. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, Z.B.; Wang, X.D.; Zheng, Y.S. Ultrasonic bonding for thermoplastic microfluidic devices without energy director. Microelectron. Eng. 2010, 87, 2429–2436. [Google Scholar] [CrossRef]
- Sun, Y.B.; Luo, Y.; Wang, X.D.; Zhang, M.M.; Feng, Y.Q. A new ultrasonic precise bonding method with ultrasound propagation feedback for polymer MEMS. Microelectron. Eng. 2011, 88, 3049–3053. [Google Scholar] [CrossRef]
- Sun, Y.B.; Wang, F.; Yang, X.H. Theoretical and Experimental Study on Vibration Propagation in PMMA Components in Ultrasonic Bonding Process. Micromachines 2017, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Vidal, E.; Quintana, I.; Etxarri, J.; Azkorbebeitia, U.; Otaduy, D.; Gonzalez, F.; Moreno, F. Optical design and development of a fiber coupled high-power diode laser system for laser transmission welding of plastics. Opt. Eng. 2012, 51, 124301. [Google Scholar] [CrossRef]
- Jiang, X.; Chandrasekar, S.; Wang, C.H. A laser microwelding method for assembly of polymer based microfluidic devices. Opt. Laser Eng. 2015, 66, 98–104. [Google Scholar] [CrossRef]
- Podbiel, D.; Boecking, L.; Bott, H.; Kassel, J.; Czurratis, D.; Laermer, F.; Zengerle, R.; Hoffmann, J. From CAD to microfluidic chip within one day: Rapid prototyping of lab-on-chip cartridges using generic polymer parts. J. Micromech. Microeng. 2020, 30, 115012. [Google Scholar] [CrossRef]
- Volpe, A.; Di Niso, F.; Gaudiuso, C.; De Rosa, A.; Vazquez, R.M.; Ancona, A.; Lugara, P.M.; Osellame, R. Welding of PMMA by a femtosecond fiber laser. Opt. Express 2015, 23, 4114–4124. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.L.; Esen, C.; Hellmann, R. A New Approach to Seal Polymer Microfluidic Devices Using Ultrashort Laser Pulses. J. Laser Micro Nanoeng. 2019, 14, 49–53. [Google Scholar] [CrossRef]
- Salvo, P.; Verplancke, R.; Bossuyt, F.; Latta, D.; Vandecasteele, B.; Liu, C.; Vanfleteren, J. Adhesive bonding by SU-8 transfer for assembling microfluidic devices. Microfluid. Nanofluid. 2012, 13, 987–991. [Google Scholar] [CrossRef]
- Dang, F.; Shinohara, S.; Tabata, O.; Yamaoka, Y.; Kurokawa, M.; Shinohara, Y.; Ishikawa, M.; Baba, Y. Replica multichannel polymer chips with a network of sacrificial channels sealed by adhesive printing method. Lab Chip 2005, 5, 472–478. [Google Scholar] [CrossRef]
- Matellan, C.; Del Rio Hernandez, A.E. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, N.Q.; Rojanasakul, Y.; Liu, Y.X. Selective stamp bonding of PDMS microfluidic devices to polymer substrates for biological applications. Sens. Actuators A Phys. 2013, 193, 186–192. [Google Scholar] [CrossRef]
- Chen, P.C.; Liu, Y.M.; Chou, H.C. An adhesive bonding method with microfabricating micro pillars to prevent clogging in a microchannel. J. Micromech. Microeng. 2016, 26, 45003. [Google Scholar] [CrossRef]
- Cheon, J.; Kim, S. Intermediate layer-based bonding techniques for polydimethylsiloxane/digital light processing 3D-printed microfluidic devices. J. Micromech. Microeng. 2019, 29, 95005. [Google Scholar] [CrossRef]
- Riegger, L.; Strohmeier, O.; Faltin, B.; Zengerle, R.; Koltay, P. Adhesive bonding of microfluidic chips: Influence of process parameters. J. Micromech. Microeng. 2010, 20, 87003. [Google Scholar] [CrossRef]
- Kratz, S.R.A.; Eilenberger, C.; Schuller, P.; Bachmann, B.; Spitz, S.; Ertl, P.; Rothbauer, M. Characterization of four functional biocompatible pressure-sensitive adhesives for rapid prototyping of cell-based lab-on-a-chip and organ-on-a-chip systems. Sci. Rep. 2019, 9, 9287. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Fazeli, A.; Moghaddam, S. Scalable bonding of nanofibrous polytetrafluoroethylene (PTFE) membranes on microstructures. J. Micromech. Microeng. 2018, 28, 15001. [Google Scholar] [CrossRef]
- Gong, X.Q.; Yi, X.; Xiao, K.; Li, S.; Kodzius, R.; Qin, J.H.; Wen, W.J. Wax-bonding 3D microfluidic chips. Lab Chip 2010, 10, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Le, N.X.T.; Lee, N.Y. Chitosan-polydopamine hydrogel complex: A novel green adhesion agent for reversibly bonding thermoplastic microdevice and its application for cell-friendly microfluidic 3D cell culture. Lab Chip 2020, 20, 3524–3534. [Google Scholar] [CrossRef]
- Zhou, X.C.; Sjoberg, R.; Druet, A.; Schwenk, J.M.; van der Wijngaart, W.; Haraldsson, T.; Carlborg, C.F. Thiol-ene-epoxy thermoset for low-temperature bonding to biofunctionalized microarray surfaces. Lab Chip 2017, 17, 3672–3681. [Google Scholar] [CrossRef]
- Song, I.H.; Park, T. PMMA Solution Assisted Room Temperature Bonding for PMMA–PC Hybrid Devices. Micromachines 2017, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Wang, H.; Liu, S.; Liu, J.; Gao, K.; Zhang, Y. Rapid prototyping of shrinkable BOPS-based microfluidic devices. Microfluid. Nanofluid. 2018, 22, 1–7. [Google Scholar] [CrossRef]
- Ku, X.Y.; Zhuang, G.S.; Li, G. A universal approach for irreversible bonding of rigid substrate-based microfluidic devices at room temperature. Microfluid. Nanofluid. 2018, 22, 1–9. [Google Scholar] [CrossRef]
- Lu, C.M.; Lee, L.J.; Juang, Y.J. Packaging of microfluidic chips via interstitial bonding. Electrophoresis 2008, 29, 1407–1414. [Google Scholar] [CrossRef]
- Lai, S.; Cao, X.; Lee, L.J. A Packaging Technique for Polymer Microfluidic Platforms. Anal. Chem. 2004, 76, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yu, S.F.; Lu, M.; Zuo, L. Microfabrication of plastic-PDMS microfluidic devices using polyimide release layer and selective adhesive bonding. J. Micromech. Microeng. 2017, 27, 55015. [Google Scholar] [CrossRef]
- Li, J.M.; Liang, C.; Zhang, H.; Liu, C. Reliable and high quality adhesive bonding for microfluidic devices. Micro Nano Lett. 2017, 12, 90–94. [Google Scholar] [CrossRef]
- Tsao, C.W.; Syu, W.C. Bonding of thermoplastic microfluidics by using dry adhesive tape. RSC Adv. 2020, 10, 30289–30296. [Google Scholar] [CrossRef]
- Huang, F.-C.; Chen, Y.-F.; Lee, G.-B. CE chips fabricated by injection molding and polyethylene/thermoplastic elastomer film packaging methods. Electrophoresis 2007, 28, 1130–1137. [Google Scholar] [CrossRef]
- Tan, H.Y.; Loke, W.K.; Nguyen, N.T. A reliable method for bonding polydimethylsiloxane (PDMS) to polymethylmethacrylate (PMMA) and its application in micropumps. Sens. Actuators B Chem. 2010, 151, 133–139. [Google Scholar] [CrossRef] [Green Version]
- You, J.B.; Min, K.I.; Lee, B.; Kim, D.P.; Im, S.G. A doubly cross-linked nano-adhesive for the reliable sealing of flexible microfluidic devices. Lab Chip 2013, 13, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Godino, N.; Gorkin, R., 3rd; Bourke, K.; Ducree, J. Fabricating electrodes for amperometric detection in hybrid paper/polymer lab-on-a-chip devices. Lab Chip 2012, 12, 3281–3284. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Polydimethylsiloxane (PDMS) irreversible bonding to untreated plastics and metals for microfluidics applications. APL Mater. 2019, 7, 81108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Gao, K.X.; Fan, Y.Q. Application of a new UV curable adhesive for rapid bonding in thermoplastic-based microfluidics. Micro Nano Lett. 2019, 14, 211–214. [Google Scholar] [CrossRef]
- Le, N.X.T.; Trinh, K.T.L.; Lee, N.Y. Poly(acrylic acid) as an adhesion promoter for UV-assisted thermoplastic bonding: Application for the in vitro construction of human blood vessels. Mater. Sci. Eng. C Mater. 2021, 122, 111874. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Trinh, K.T.L.; Lee, N.Y. Heat and pressure-resistant room temperature irreversible sealing of hybrid PDMS-thermoplastic microfluidic devices via carbon-nitrogen covalent bonding and its application in a continuous-flow polymerase chain reaction. RSC Adv. 2020, 10, 16502–16509. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, R.; Lee, N.Y. Chemically robust succinimide-group-assisted irreversible bonding of poly(dimethylsiloxane)-thermoplastic microfluidic devices at room temperature. Analyst 2020, 145, 6887–6894. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Liu, K.; Chen, H.; Nishida, T.; Fan, Z.H. Chemical-assisted bonding of thermoplastics/elastomer for fabricating microfluidic valves. Anal. Chem. 2011, 83, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Yue, C.Y.; Venkatraman, S.S.; Ma, L.L. Low-temperature (below T-g) thermal bonding of COC microfluidic devices using UV photografted HEMA-modified substrates: High strength, stable hydrophilic, biocompatible surfaces. J. Mater. Chem. 2011, 21, 15031–15040. [Google Scholar] [CrossRef]
- Yu, H.; Chong, Z.Z.; Tor, S.B.; Liu, E.; Loh, N.H. Low temperature and deformation-free bonding of PMMA microfluidic devices with stable hydrophilicity via oxygen plasma treatment and PVA coating. RSC Adv. 2015, 5, 8377–8388. [Google Scholar] [CrossRef]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.E.; Ahn, S.K.; Kang, J.H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef]
- Hoang, M.V.; Chung, H.J.; Elias, A.L. Irreversible bonding of polyimide and polydimethylsiloxane (PDMS) based on a thiol-epoxy click reaction. J. Micromech. Microeng. 2016, 26, 105019. [Google Scholar] [CrossRef] [Green Version]
- Yussuf, A.A.; Sbarski, I.; Hayes, J.P.; Solomon, M.; Tran, N. Microwave welding of polymeric-microfluidic devices. J. Micromech. Microeng. 2005, 15, 1692–1699. [Google Scholar] [CrossRef]
- Lei, K.F.; Ahsan, S.; Budraa, N.; Li, W.J.; Mai, J.D. Microwave bonding of polymer-based substrates for potential encapsulated micro/nanofluidic device fabrication. Sens. Actuators A 2004, 114, 340–346. [Google Scholar] [CrossRef]
- Toossi, A.; Moghadas, H.; Daneshmand, M.; Sameoto, D. Bonding PMMA microfluidics using commercial microwave ovens. J. Micromech. Microeng. 2015, 25, 85008. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Sbarski, I.; Solomon, M.; Tran, N.; Hayes, J.P. Sealing of polymeric-microfluidic devices by using high frequency electromagnetic field and screen printing technique. J. Mater. Process. Technol. 2007, 189, 401–408. [Google Scholar] [CrossRef]
- Holmes, R.J.; McDonagh, C.; McLaughlin, J.A.D.; Mohr, S.; Goddard, N.J.; Fielden, P.R. Microwave bonding of poly(methylmethacrylate) microfluidic devices using a conductive polymer. J. Phys. Chem. Solids 2011, 72, 626–629. [Google Scholar] [CrossRef] [Green Version]
- Mani, K.B.; Hossan, M.R.; Dutta, P. Thermal analysis of microwave assisted bonding of poly(methyl methacrylate) substrates in microfluidic devices. Int. J. Heat Mass Trans. 2013, 58, 229–239. [Google Scholar] [CrossRef]








| Thermoplastic Materials | Physical Properties | Chemical Resistance | Optical Transmissivity | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Young’s Modulus | Tg 1 | Tm 2 | CTE 3 | Solvent | Acid/Base | Visible | UV | ||
| Polymethylmethacrylate (PMMA) | 3200 | 104–105 | 130 | 6–8 | good | good | excellent | good | [13] |
| Polystyrene (PS) | 2300–4100 | 80–90 | 240–260 | 10–150 | fair | good | excellent | fair | [13] |
| Cyclic olefin polymers (COC/COP) | 2600–3200 | 65–170 | 190–320 | 60–80 | excellent | good | excellent | excellent | [14] |
| Polycarbonate (PC) | 2300–2700 | 145–148 | 260–270 | 60–70 | good | good | excellent | fair | [15] |
| Bonded Pairs | Parameters | Tools and Experimental Setup | Surface Treatment | Bonding Result | Reference | ||
|---|---|---|---|---|---|---|---|
| T °C | Pressure | Time | |||||
| PMMA-PMMA | 90 | ∼5.5 kg/cm2 | 10 min | Spring-driven press | Surface treatment with 5% dibutyl phthalate (DBP) in isopropanol | - | [31] |
| ∼140 | ∼6 kg/cm2 | 10 min | Positive temperature coefficient ceramic heater and spring-driven press | Cured with epoxy | - | [32] | |
| 140 | 2.2 MPa | 41 min | Steel plates | 82.3 °C isopropyl alcohol for 75 s | 185.0 ± 33.3 kPa, and 808.0 ± 80 kPa for untreated and treated samples | [51] | |
| 91–93 | 1.4–1.9 MPa | 360 s | Home-made hot embossing apparatus | Water pretreatment for 1 h | Bonding rate of 96.8% | [49] | |
| 160 | 1.38 MPa | 1 min | GATB using Nanoimprint lithography (NIL) apparatus | Oxygen plasma treatment for 1 min | Failure load 1670 g for GATB at 160 °C and 1.38 MPa | [38] | |
| 95 | 1–2 MPa | 3 min | Interference-assisted bonding with hot embossing equipment | - | - | [50] | |
| 120 | Low pressure | 1 h | High temperature oven treatment and vacuum dried at 80 °C | - | Microchannels with very low aspect ratios (AR = 1:100) | [39] | |
| PMMA-COC | 70 | 680 kN/m2 | 900 s | Placed in vacuum seal between thermal embosser | Oxygen plasma treatment | Bond strength 67 ± 7 mJ/cm2 | [46] |
| PMMA-TPE | 70 | 1.6 MPa | 15 min | Pneumatic hot press and electronic pressure regulator | UV surface treatment | Burst load >100 N | [44] |
| 80 | 0.52 MPa | - | Hot press machine | Plasma treatment for 1 min | Bonding strength 16 N/cm2 | [33] | |
| PS-PS | 105 | 0.4 MPa | 5 min | Nanostructured plate on PS | - | Deformation ratio 1.1% | [36] |
| 93.3 | 6.9 MPa | 10 min | Hot press machine | Rinsed with isopropyl alcohol and deionized water | Bonding strength 375.5 kPa | [30] | |
| PI-PI | 380–390 | 100 N | 3–5 min | Ceramic heater | - | Bonding strength 80 N | [40] |
| PET-PET | 50 | 0.15 MPa | 15 min | Hot embossing machine | O2 Plasma and ethanol treatment | Bonding strength 0.424 MPa | [45] |
| COC-eCOC | 80 | 2 bar | 10 min | Conventional hot press | UV/Ozone treatment for 10 min | Bond strength 445 J/m2 | [34] |
| Bonded Pairs | Solvent Used | Parameters | Tools and Experimental Setup | Surface Treatment | Bonding Result | Reference | ||
|---|---|---|---|---|---|---|---|---|
| T °C | Pressure | Time | ||||||
| PMMA-PMMA | Pure isopropyl alcohol | 70 | No pressure | 10 s | Spin coater at 2000 rpm | - | - | [71] |
| Chloroform | 20 | 1 atm | 12 min | Exposed to CHCl3 vapour | O2 plasma treatment | Bond strength 38 MPa for double sided exposure | [77] | |
| Chloroform-Ethanol VC:VE = 1:10 | 40 | - | 10 min | Soak bonding method | - | Bonding strength 267.5 N/cm2 | [54] | |
| Dichloromethane, isopropanol (v:v 2:8) | - | - | 10 s | Precision needle-tip applicator | Corona Treatment | Bond strength 2.208 ± 0.001 MPa | [60] | |
| Chloroform vapour | - | - | 10 s | Vapor solvent bonding | UV irradiation | Failure load 3200 N | [74] | |
| Dichlororethane | - | ∼0.2 kg/cm2 | 2 min | Applied by capillary effect using syringe | Cleaned with water and isopropanol | Bond strength 12 MPa | [63] | |
| Acetic acid | - | - | - | Activated using microwave for 2 min 50 s | - | Bond strength 14.95 ± 0.77 MPa | [65] | |
| Ethanol (95%) | - | - | 56 s | Spin coating at 190 rpm for 10 s | UV irradiation | Bond strength > 10 bar | [69] | |
| Ethanol | 68 | 120 kPa | 15 min | Heated in a fan-assisted oven | Rinsed with isopropyl alcohol and deionized water | Bonding Strength 28.5 MPa | [67] | |
| COP-COP | Cyclohexane | 30 | 3 kN | 3 min | Hot Press time of 5 min at 90 °C | - | Microchannel coeficient of variance (CV) 1.4% | [56] |
| Dichloromethane | 30 | 1 kN | 1 min 30 s | Hot Press time of 5 min at 90 °C | - | CV < 1% | [56] | |
| Toluene | 30 | 1 kN | 4 min 30 s | Hot Press time of 5 min at 90 °C | - | CV < 1% | [56] | |
| PMMA-PS | Acetone with DI water | 40 | 103 kPa | 20 min | Pipette and pre-heated hotplate | Rinsed in DI water | Bonding strength 34.4 J/m2 for 80% acetone | [66] |
| PMMA-ABS | Ethanol Solution | - | - | - | Spray coating | UV exposure for 84 s and post annealing at 55 °C | - | [72] |
| Bonded Pairs | Parameters | Tools and Experimental Setup | Bonding Result | Reference |
|---|---|---|---|---|
| Ultrasonic Welding | ||||
| PMMA-PMMA | Ultrasonic cleaner Power 300 W, 40 kHz with ultrasound intensity of 0.05 W cm−3 | Assisted by ethyl alcohol solvent vaporized at 45 °C for 10 min | Bond strength 30.9 mJ cm−2 at 60 °C No deformation at 60 °C | [102] |
| Frequency 30 kHz speed 50 mm/s Pressure 0.16 MPa time 30 s | Preheating at temperature 75°C | Tensile strength 0.95 MPa | [103] | |
| frequency of 30 kHz, a power of 1000 W and a maximum amplitude of 60 μm | Ultrasonic welding system (Branson 2000X f/aef), | Burst pressure: 680 kPa | [106] | |
| Ultrasonic welder 1500 W at 20 kHz, ultrasonic amplitude 60 μm; holding time 5 s Bonding pressure: 24–60 kgf | Self-Balancing jig and energy director | Bonding strength > 2.5 MPa | [107] | |
| Ultrasonic generator with 20 KHz frequency amplitude 45 μm, 2 layer pressure: 0.25 MPa time 0.6 s 5 layer Pressure 0.45 MPa and time 1 s | Ultrasonic bonding system (Dizo-ultrasonic NC-1800P) | Burst Pressure for two layer linear and serpentine channel and five layer: 553 ± 48 kPa, 572 ± 52 kPa and 417 ± 62 kPa respectively | [109] | |
| Preheating temperature (°C) 70 Amplitude (μm) 6.6 Trigger pressure (MPa) 0.032 Ultrasonic time (s) 25 Ultrasonic pressure (MPa) 0.276 Holding time (s) 5 Holding pressure (MPa) 0.147 | Ultrasonic welding machine (Branson 2000X f/aef, Branson, MI, USA), fixture and hot plate Thermal assisted ultrasonic bonding | tensile strength of 0.95 MPa Dimension loss 0.66% ± 0.60 | [110] | |
| Amplitude (μm) 7.2 Trigger pressure (MPa) 0.033 Ultrasonic time (s) 10 Ultrasonic pressure (MPa) 0.297 Holding time (s) 5 Holding pressure (MPa) 0.297 | Ultrasonic welding machine (Branson 2000X f/aef, Branson, MI, USA) Solvent assisted ultrasonic bonding Isopropyl alcohol as solvent | tensile strength 2.25 MPa Dimension loss 0.58% ± 0.55 | [110] | |
| Ultrasonic welder power 2 kW, clamping force 28 kN, Frequency 20 kHz | Ultrasonic welder | No blockage and can withstand 6 bars (gauge) pressure for at least 10 min. | [108] | |
| COP-COP | Ultrasonic welder of Power 750 W frequency 35 kHz | Preheating at 60 °C | -- | [97] |
| Ultrasonic bonder of frequency 20 kHz, speed 20 mm/s, 90% amplitude for 0.1 s Pressure applied for 10 s | Ultrasonic bonder (Branson, 2000X-aef, USA) Self-balancing jig | No leakage | [100] | |
| Laser Welding | ||||
| PMMA-PMMA | laser power 25 W beam intensity 70 W/cm2 processing time of 15 s output at 970 nm | High power CW diode laser system (LDM 100, Laserlines, Germany) PMMA substrates deposited with titanium film | Tensile strength 6 Mpa | [115] |
| Ultrafast fiber laser at a wavelength of 1030 nm and a repetition rate of 5 MHz shortest pulse duration of 650 fs, | Ultrafast fiber laser amplifier | Leakage test upto 1 bar | [117] | |
| PC-TPE | continous wave fiber laser working at a wavelength of 1064 nm | Contour laser welding system (Novolas WS AT from Leister Technologies AG) carbon black particles incorporated in TPE | Average peel strength greater than 0.9 Nmm−1 | [116] |
| COC-COC | fundamental wavelength of 1028 nm shortest pulse duration 220 fs pulse repetition rate 610 kHz | Ultrashort pulse laser (Light Conversion, Pharos-10-600) | Leakage test upto 0.6 Mpa for 30 min | [118] |
| Bonded Pairs | Adhesive Used | Curing Method | Coating Method | Surface Treatment | Bonding Result | Reference |
|---|---|---|---|---|---|---|
| PMMA-PMMA | Chitosan (CS)-Polydopamine (pDA) hydrogel(2:1) | UV irradiation (234 nm, 135 mW cm−2) | Using Micropipette | O2 plasma treatment | 0.7 MPa for 60 s UV exposure and applicable for reversible bonding | [129] |
| UV curable (LOCTITE AA 3311) (Acrylated urethane) | UV exposure of 1800 μW/cm2 with the peak at 365 nm | Spin coating at 500 rpm thickness around 10 μm | Ultrasonic cleaning | ∼1.35 MPa for UV exposure of 30 s | [145] | |
| Epoxy resin (Araldite Standard) | Cured overnight at room temperature | Capillery driven adhesive | acetone followed by a heat treatment at 70 °C for 15 min | 200 ± 92 kPa when cured for 72 h | [121] | |
| PET film with silicone adhesive and UV curable adhesive | UV curing | Coated into surface | - | 364 ± 7 kPa burst pressure | [133] | |
| UV curable adhesive | UV irradiation for 60 s and vacuum bagging method for uniform pressure | Spin coating at 500 rpm for 10 s followed by 1500 rpm for 20 s | PMMA cleaned with diluted isopropyl alcohol (IPA) | Burst Pressure 10 bar | [123] | |
| Polyacrylic acid | UV irradiation (234 nm, 135 mW cm−2) | Pippette | - | Bond Strength 1.18 Mpa for 60 s UV exposure | [146] | |
| PDMS-PS | PrimeCoat-Epoxy adhesive layer | Cured by heating in oven at 60 °C for 3 h | Selective stamp coating | Oxygen plasma treatment for 30 s | maximum shear stress 2000 Pa | [122] |
| PMMA-PC | 2.5% (w/w) polymethyl methacrylate (PMMA) solution | dissolved in propylene glycol monomethyl ether acetate (PGMEA) | Spin coated | Annealed in an oven at 80 °C | Bonding strength 0.721 ± 0.03 MPa | [131] |
| PDMS-PI | Epoxy adhesive | Cured in hotplate at 60 °C for 2 h | Stamp and stick | PDMS treated with oxygen plasma for 30 s | Peeling force 5 N Bonding strength 100 kPa | [136] |
| PDMS-PMMA | ARclear® Optically clear adhesive 8154 | Thermal curing at 80 °C for 1 h followed by oxygen plasma | Spin coating at 1500 rpm for 30 s | Washed with ethanol and deionised water | Bond strength > 20 kPa | [140] |
| COC-COC | ORDYL photoresist | baked for 2 min at 80 °C on a hotplate | Manually laminated | oxygen plasma treatment for 4 min | shear strength 28 MPa | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, K.; Tsao, C.-W. Recent Advances in Thermoplastic Microfluidic Bonding. Micromachines 2022, 13, 486. https://doi.org/10.3390/mi13030486
Giri K, Tsao C-W. Recent Advances in Thermoplastic Microfluidic Bonding. Micromachines. 2022; 13(3):486. https://doi.org/10.3390/mi13030486
Chicago/Turabian StyleGiri, Kiran, and Chia-Wen Tsao. 2022. "Recent Advances in Thermoplastic Microfluidic Bonding" Micromachines 13, no. 3: 486. https://doi.org/10.3390/mi13030486
APA StyleGiri, K., & Tsao, C. -W. (2022). Recent Advances in Thermoplastic Microfluidic Bonding. Micromachines, 13(3), 486. https://doi.org/10.3390/mi13030486