Simple and Label-Free Detection of Carboxylesterase and Its Inhibitors Using a Liquid Crystal Droplet Sensing Platform
Abstract
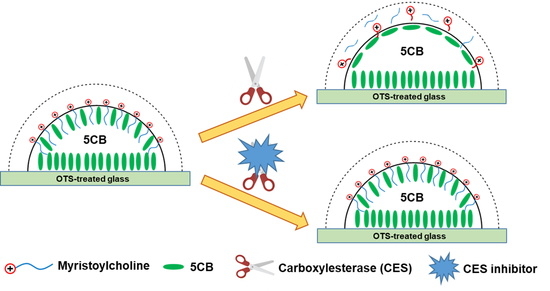
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Preparation of Octyltrichlorosilane (OTS)-Modified Glass Slides
2.3. Preparation of LC Droplet Patterns and Fabrication of LC Cells
2.4. Preparation of the Aqueous Solution
3. Results and Discussion
3.1. Optimization of the Myr Concentration
3.2. Feasibility and Detection Limit of the LC Droplet-Based Biosensor for CES Detection
3.3. Specificity of the LC Droplet-Based Biosensor in CES Detection
3.4. Detection of CES in Human Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Pina, A.S.; Roque, A.C.A. Bio-recognition and detection using liquid crystals. Biosens. Bioelectron. 2009, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Xue, C.Y.; Yang, K.L.; Yung, L.Y.L. Decorating liquid crystal surfaces with proteins for real-time detection of specific protein–protein binding. Adv. Funct. Mater. 2009, 19, 3574–3579. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Q.; Tian, T.; Yu, L. Simple and sensitive detection of pesticides using the liquid crystal droplet patterns platform. Sens. Actuators B Chem. 2017, 238, 676–682. [Google Scholar] [CrossRef]
- Liao, S.; Qiao, Y.; Han, W.; Xie, Z.; Wu, Z.; Shen, G.; Yu, R. Acetylcholinesterase liquid crystal biosensor based on modulated growth of gold nanoparticles for amplified detection of acetylcholine and inhibitor. Anal. Chem. 2012, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441. [Google Scholar] [CrossRef]
- Rouhbakhsh, Z.; Verdian, A.; Rajabzadeh, G. Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline. Talanta 2020, 206, 120246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Q.; Guo, Y.; Yu, L. A cationic surfactant-decorated liquid crystal sensing platform for simple and sensitive detection of acetylcholinesterase and its inhibitor. Biosens. Bioelectron. 2015, 72, 25–30. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Qi, L.; Wang, Q.; Yu, L.; Lin, J.M.; Hu, Q. Screening of Xanthine Oxidase Inhibitors by Liquid Crystal-Based Assay Assisted with Enzyme Catalysis-Induced Aptamer Release. Anal. Chem. 2021, 93, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- McUmber, A.C.; Noonan, P.S.; Schwartz, D.K. Surfactant–DNA interactions at the liquid crystal–aqueous interface. Soft Matter 2012, 8, 4335–4342. [Google Scholar] [CrossRef]
- Munir, S.; Park, S.Y. Liquid crystal-based DNA biosensor for myricetin detection. Sens. Actuators B Chem. 2016, 233, 559–565. [Google Scholar] [CrossRef]
- Liu, D.; Jang, C.H. A new strategy for imaging urease activity using liquid crystal droplet patterns formed on solid surfaces. Sens. Actuators B Chem. 2014, 193, 770–773. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, C.H. Optical imaging of cholylglycine by using liquid crystal droplet patterns on solid surfaces. J. Mater. Sci. 2016, 51, 2033–2040. [Google Scholar] [CrossRef]
- Di, L. The impact of carboxylesterases in drug metabolism and pharmacokinetics. Curr. Drug Metab. 2019, 20, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Feng, D.; Shi, W.; Li, X.; Ma, H. A spectroscopic off-on probe for simple and sensitive detection of carboxylesterase activity and its application to cell imaging. Analyst 2012, 137, 716. [Google Scholar] [CrossRef]
- Li, W.; Zhai, C.; Wang, S.; Huang, W.; Liu, Y.; Li, Z. Detection of carboxylesterase by a novel hydrosoluble near-infrared fluorescence probe. RSC Adv. 2019, 9, 40689. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Lee, E.Y.; Lee, H.J.; Kim, K.Y.; Lee, H.; Jeong, S.K.; Jeong, A.S.; Cho, S.Y.; Kim, S.A.; Song, S.Y.; et al. Human plasma carboxylesterase 1, a novel serologic biomarker candidate for hepatocellular carcinoma. Proteomics 2009, 9, 3989–3999. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, Y.; Hou, Y.; Zhong, G.; Gao, R.; Wu, J.; Shen, B.; Zhang, X. Detection of carboxylesterase 1 and carbamates with a novel fluorescent protein chromophore based probe. Dyes Pigment. 2021, 192, 109444. [Google Scholar] [CrossRef]
- Koitka, M.; Hochel, J.; Obst, D.; Rottmann, A.; Gieschen, H.; Borchert, H.H. Determination of rat serum esterase activities by an HPLC method using S-acetylthiocholine iodide and p-nitrophenyl acetate. Anal. Biochem. 2008, 381, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, T.; Schweppe, F.; Krebs, B.; Karst, U. A tripod ligand as new sensitiser for the enzyme amplified lanthanide luminescence determination of esterase. Analyst 2003, 128, 29. [Google Scholar] [CrossRef]
- Wang, D.D.; Jin, Q.; Zou, L.W.; Hou, J.; Lv, X.; Lei, W.; Cheng, H.L.; Ge, G.B.; Yang, L. A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples. Chem. Commun. 2016, 52, 3183–3186. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, Q.; Fang, M.; Yu, L. Label-free, rapid, and sensitive detection of carboxylesterase using surfactant-doped liquid crystal sensor. J. Mol. Liq. 2019, 296, 111921. [Google Scholar] [CrossRef]
- Brake, J.M.; Abbott, N.L. An Experimental System for Imaging the Reversible Adsorption of Amphiphiles at Aqueous-Liquid Crystal Interfaces. Langmuir 2002, 18, 6101–6109. [Google Scholar] [CrossRef]
- Duong, T.D.S.; Jang, C.H. A label-free liquid crystal droplet-based sensor used to detect lead ions using single-stranded DNAzyme. Colloid Surface A 2020, 604, 125304. [Google Scholar] [CrossRef]
- Jin, Q.; Feng, L.; Wang, D.D.; Wu, J.J.; Hou, J.; Dai, Z.R.; Sun, S.G.; Wang, J.Y.; Ge, G.B.; Cui, J.N.; et al. A highly selective near-infrared fluorescent probe for carboxylesterase 2 and its bioimaging applications in living cells and animals. Biosens. Bioelectron. 2016, 83, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.W.; Jin, Q.; Wang, D.D.; Qian, Q.K.; Hao, D.C.; Ge, G.B.; Yang, L. Carboxylesterase inhibitors: An update. Curr. Med. Chem. 2018, 25, 1627–1649. [Google Scholar] [CrossRef]
- Wadkins, R.M.; Hyatt, J.L.; Wei, X.; Yoon, K.J.P.; Wierdl, M.; Edwards, C.C.; Morton, C.L.; Obenauer, J.C.; Damodaran, K.; Beroza, P.; et al. Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J. Med. Chem. 2005, 48, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.D.; Bencharit, S.; Edwards, C.C.; Hyatt, J.L.; Tsurkan, L.; Bai, F.; Fraga, C.; Morton, C.L.; Howard-Williams, E.L.; Potter, P.M.; et al. Structural Insights into Drug Processing by Human Carboxylesterase 1: Tamoxifen, Mevastatin, and Inhibition by Benzil. J. Mol. Biol. 2005, 352, 165–177. [Google Scholar] [CrossRef]






| Methods | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Spectroscopic probe sensor | 40–300 U/L | 0.086 U/L | [14] |
| Near-infrared fluorescence probe | 10–300 U/L | 3.4 U/L | [15] |
| Bioluminescence sensor | 0.01–6 mg/L | 0.01 mg/L | [20] |
| Surfactant-doped LC-based sensor a | 18–180 U/L | 1 mg/L (18 U/L) | [21] |
| LC droplet-based biosensor | - | 0.1 mg/L (2.8 U/L) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.-K.; Jang, C.-H. Simple and Label-Free Detection of Carboxylesterase and Its Inhibitors Using a Liquid Crystal Droplet Sensing Platform. Micromachines 2022, 13, 490. https://doi.org/10.3390/mi13030490
Nguyen D-K, Jang C-H. Simple and Label-Free Detection of Carboxylesterase and Its Inhibitors Using a Liquid Crystal Droplet Sensing Platform. Micromachines. 2022; 13(3):490. https://doi.org/10.3390/mi13030490
Chicago/Turabian StyleNguyen, Duy-Khiem, and Chang-Hyun Jang. 2022. "Simple and Label-Free Detection of Carboxylesterase and Its Inhibitors Using a Liquid Crystal Droplet Sensing Platform" Micromachines 13, no. 3: 490. https://doi.org/10.3390/mi13030490
APA StyleNguyen, D. -K., & Jang, C. -H. (2022). Simple and Label-Free Detection of Carboxylesterase and Its Inhibitors Using a Liquid Crystal Droplet Sensing Platform. Micromachines, 13(3), 490. https://doi.org/10.3390/mi13030490