Fabrication of a Disposable Amperometric Sensor for the Determination of Nitrite in Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
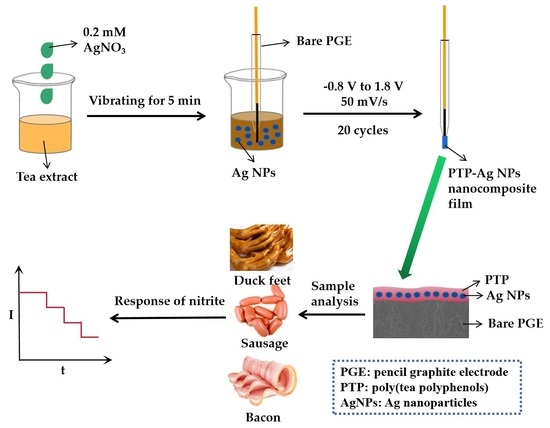
2.2. Green Synthesis of AgNPs Using Tea Extract
2.3. Preparation of Different Electrodes
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of AgNPs
3.2. Morphological Characterization of Different Electrodes
3.3. Electrochemical Impedance Spectroscopy Characterization of Different Electrodes
3.4. Determination of Nitrite with Different Electrodes
3.4.1. Electrochemical Responses of Nitrite on Different Electrodes
3.4.2. Electrocatalysis Mechanisms of PTP-AgNP Film
3.4.3. Performances of the Nitrite Sensor
3.4.4. Measurement of Nitrite in Food Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Golden, M.C.; Wanless, B.J.; David, J.R.D.; Kottapalli, B.; Lineback, D.S.; Talley, R.J.; Glass, K.A. Effect of Cultured Celery Juice, Temperature, and Product Composition on the Inhibition of Proteolytic Clostridium botulinum Toxin Production. J. Food Prot. 2017, 80, 1259–1265. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Jardim, E.O.; Ramos–Fernandez, E.V.; Villora-Picó, J.J.; Pastor–Blas, M.M.; Silvestre-Albero, J. Highly N2–Selective Activated Carbon-Supported Pt-In Catalysts for the Reduction of Nitrites in Water. Front. Chem. 2021, 9, 733881. [Google Scholar] [CrossRef] [PubMed]
- Salhi, O.; Ez-Zine, T.; Oularbi, L.; El Rhazi, M. Electrochemical Sensing of Nitrite Ions Using Modified Electrode by Poly 1,8–Diaminonaphthalene/Functionalized Multi–Walled Carbon Nanotubes. Front. Chem. 2022, 10, 870393. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Gürkan, R.; Olgaç, E. Development of a New Methodology for Indirect Determination of Nitrite, Nitrate, and Total Nitrite in the Selected Two Groups of Foods by Spectrophotometry. Food Anal. Methods 2017, 10, 2194–2206. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, Y.R.; Huang, C.X.; Chen, T.Y.; Wu, J. Ultra–Weak Chemiluminescence Enhanced by Cerium–Doped LaF3 Nanoparticles: A Potential Nitrite Analysis Method. Front. Chem. 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Di, T.A.; Vita, V.; Berardi, G.; Iammarino, M. Development and Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Products by Capillary Ion Chromatography (CIC). Food. Anal. Method. 2019, 12, 1813–1822. [Google Scholar] [CrossRef]
- Tembo, Z.N.; Aygun, S.F. Capillary electrophoretic method for the simultaneous determination of nitrate, nitrite and bromate ions in some selected plants. J. Sci. Food. Agric. 2021, 101, 5391–5397. [Google Scholar] [CrossRef]
- Wang, P.Y.; Zhang, Y.; Liu, Y.L.; Pang, X.P.; Liu, P.; Dong, W.F.; Mei, Q.; Qian, Q.; Li, L.; Yan, R.H. Starch–Based Carbon Dots for Nitrite and Sulfite Detection. Front. Chem. 2021, 9, 782238. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lin, X.G.; Peng, Z.J.; Zhou, Y.; Xu, S.B.; Song, M.; Jin, L.F.; Zheng, X.D. A Fast and Highly Selective Nitrite Sensor Based on Interdigital Electrodes Modified with Nanogold Film and Chrome–Black T. Front. Chem. 2020, 8, 366. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Y.; Qin, R.; Xu, H.; Ye, M.; Nie, P. MoS2/MWCNT-COOH-Modified Glassy Carbon Electrode for Nitrite Detection in Water Environment. Chemosensors 2022, 10, 419. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, D.S.; Wang, C.H.; Zhang, W.; Liu, C.; Zhang, K.Y. Graphene Oxide-Gold Nanostars Modified Glassy Carbon Electrode for Highly Efficient Sensing for Nitrite. Int. J. Electrochem. Sci. 2022, 17, 221218. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandra, M. Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis. J. Anal. Methods. Chem. 2017, 2017, 1905968. [Google Scholar] [CrossRef] [Green Version]
- Torrinha, Á.; Amorim, C.G.; Montenegro, M.C.; Araújo, A.N. Biosensing based on pencil graphite electrodes. Talanta 2018, 190, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Shao, H.; Badal, M.M.R.; Razeeb, K.M.; Jamal, M. Pencil graphite as electrode platform for free chlorine sensors and energy storage devices. PLoS ONE 2021, 16, e0248142. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.F.; Ferreira, A.L.; Torres, M.D.T.; de Araujo, W.R.; de la Fuente-Nunez, C. Minute-scale detection of SARS-CoV-2 using a low-cost biosensor composed of pencil graphite electrodes. Proc. Natl. Acad. Sci. USA 2021, 118, e2106724118. [Google Scholar] [CrossRef]
- Sree, V.G.; Sohn, J.I.; Im, H. Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3–Nitrophenol and 4-Nitrophenol in Environmental Water Samples. Sensors 2022, 22, 1151. [Google Scholar] [CrossRef] [PubMed]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Curcumin graphite pencil electrode modified with molybdenum disulfide nanosheets decorated gold foams for simultaneous quantification of nitrite and hydrazine in water samples. Anal. Chim. Acta 2020, 1137, 19–27. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.Y.; Li, Z.P.; Xiao, G.; Yang, Q.C.; Sun, P.; Cheng, L.Y.; Niu, W.C.; Bi, J.S.; et al. ISFET and Dex-AgNPs based portable sensor for reusable and real-time determinations of concanavalin A and glucose on smartphone. Biosens. Bioelectron. 2020, 151, 111962. [Google Scholar] [CrossRef]
- Douaki, A.; Demelash Abera, B.; Cantarella, G.; Shkodra, B.; Mushtaq, A.; Ibba, P.; Inam, A.S.; Petti, L.; Lugli, P. Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance. Nanomaterials 2020, 10, 1167. [Google Scholar] [CrossRef]
- Zhou, T.; Su, Z.; Wang, X.; Luo, M.C.; Tu, Y.F.; Yan, J.L. Fluorescence detections of hydrogen peroxide and glucose with polyethyleneimine-capped silver nanoclusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 244, 118881. [Google Scholar] [CrossRef]
- Ma, C.; Qian, Y.; Zhang, S.; Song, H.; Gao, J.; Wang, S.; Liu, M.X.; Xie, K.J.; Zhang, X.M. Temperature-controlled ethanolamine and Ag-nanoparticle dual-functionalization of graphene oxide for enhanced electrochemical nitrite determination. Sensor. Actuat. B Chem. 2018, 274, 441–450. [Google Scholar] [CrossRef]
- Salagare, S.; Adarakatti, P.S.; Yarradoddappa, V. Facile synthesis of silver nanoparticle-decorated zinc oxide nanocomposite–based pencil graphite electrode for selective electrochemical determination of nitrite. Carbon Lett. 2021, 31, 1273–1286. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, Y.C.; Chuang, W.S.; Li, J.H.; Wang, Y.S.; Chuang, C.H.; Chen, C.Y.; Kung, C.Y. Pore-Confined Silver Nanoparticles in a Porphyrinic Metal–Organic Framework for Electrochemical Nitrite Detection. ACS Appl. Nano. Mater. 2020, 3, 9440–9448. [Google Scholar] [CrossRef]
- Qing, W.X.; Chen, K.; Wang, Y.; Liu, X.H.; Lu, M.H. Green synthesis of silver nanoparticles by waste tea extract and degradation of organic dye in the absence and presence of H2O2. Appl. Surf. Sci. 2017, 423, 1019–1024. [Google Scholar] [CrossRef]
- Wang, T.W.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.H.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Polym. 2020, 23, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wadhwa, R.; Kumar, N.; Maurya, P.K. A comparative study of chemically synthesized and camellia sinensis leaf extract-mediated silver nanoparticles. 3 Biotech 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.G.; Zheng, D.Y.; Gan, T.; Hu, C.G.; Hu, S. Development and application of estradiol sensor based on layer-by-layer assembling technique. J. Exp. Nanosci. 2011, 6, 13–28. [Google Scholar] [CrossRef]
- Bajpai, M.; Srivastava, R.; Dhar, R.; Tiwari, R.S. Review on optical and electrical properties of conducting polymers. Indian J. Mater. Sci. 2016, 2016, 5842763. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.L.; Lu, G.J.; Hu, X.Y.; Li, H.B.; Ding, L.L.; Wang, Y. Electrochemical preparation of poly(bromothymol blue) film and its analytical application. J. Appl. Electrochem. 2011, 41, 143–149. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Hu, C.G.; Peng, Y.F.; Hu, S.S. A carbon nanotube/polyvanillin composite film as an electrocatalyst for the electrochemical oxidation of nitrite and its application as a nitrite sensor. Electrochim. Acta 2009, 54, 4910–4915. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kaewjunlakan, C.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Thipwimonmas, K.; Kongkaew, S.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim. Acta 2021, 377, 138072. [Google Scholar] [CrossRef]
- Ba, X.; Luo, L.Q.; Ding, Y.P.; Zhang, Z.; Chu, Y.L.; Wang, B.; Ouyang, X. Poly(alizarin red)/graphene modified glassy carbon electrode for simultaneous determination of purine and pyrimidine. Anal. Chim. Acta 2012, 752, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mutharani, B.; Ranganathan, P.; Chen, S.M. Chitosan–gold collapse gel/poly (bromophenol blue) redox-active film. A perspective for selective electrochemical sensing of flutamide. Int. J. Biol. Macromol. 2019, 124, 759–770. [Google Scholar] [CrossRef]
- Ouyang, X.Q.; Luo, L.Q.; Ding, Y.P.; Liu, B.D.; Xu, D.; Huang, A.Q. Simultaneous determination of uric acid, dopamine and ascorbic acid based on poly(bromocresol green) modified glassy carbon electrode. J. Electroanal. Chem. 2015, 748, 1–7. [Google Scholar] [CrossRef]
- Hsieh, M.T.; Whang, T.J. Mechanistic investigation on the electropolymerization of phenol red by cyclic voltammetry and the catalytic reactions toward acetaminophen and dopamine using poly(phenol red)-modified GCE. J. Electroanal. Chem. 2017, 795, 130–140. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Lajevardi, A.; Rezaei, Z.; Ghorbanpour, M.; Tanzifi, M. Ultrasonic–assisted green synthesis of silver nanoparticles using mentha aquatica leaf extract for enhanced antibacterial properties and catalytic activity. Colloid Interfac. Sci. 2020, 35, 100252. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. A comparative study on synthesis of AgNPs on cellulose nanofibers by thermal treatment and DMF for antibacterial activities. Mater. Sci. Eng. C Mater. Biol. 2019, 98, 1179–1195. [Google Scholar] [CrossRef]
- Suarez, I.J.; Otero, T.F.; Marquez, M. Diffusion coefficients in swelling polypyrrole: ESCR and Cottrell models. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Ikhsan, N.L.; Rameshkumar, P.; Pandikumar, A.; Shahid, M.M.; Huang, N.M.; Kumar, S.V.; Kim, H.N. Facile synthesis of graphene oxide–silver nanocomposite and its modified electrode for enhanced electrochemical detection of nitrite ions. Talanta 2015, 144, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.G.; Silveira, C.M.; Moura, J.J.G. Biosensing nitrite using the system nitrite redutase/Nafion/methylviologen—A voltammetric study. Biosens. Bioelectron. 2007, 22, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Menart, E.; Jovanovski, V.; Hocevar, S.B. Silver particle-decorated carbon paste electrode based on ionic liquid for improved determination of nitrite. Electrochem. Commun. 2015, 52, 45–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.H.; Yuan, S.S.; Wang, H.H.; He, C.D. Electrocatalysis and detection of nitrite on a reduced graphene/Pd nanocomposite modified glassy carbon electrode. Sens. Actuators B 2013, 185, 602–607. [Google Scholar] [CrossRef]
- Yue, R.; Lu, Q.; Zhou, Y.K. A novel nitrite biosensor based on single-layer graphene nanoplatelet–protein composite film. Biosens. Bioelectron. 2011, 26, 4436–4441. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Voiry, D.; Weisel, C.; Gow, A.; Laumbach, R.; Kipen, H.; Chhowala, M.; Javanmard, M. Toward point-of-care management of chronic respiratory conditions: Electrochemical sensing of nitrite content in exhaled breath condensate using reduced graphene oxide. Microsyst. Nanoeng. 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, P.; Shen, M.; Ning, L.; Chen, G.; Yin, Y. Functionalization of platinum nanoparticles for electrochemical detection of nitrite. Anal. Bioanal. Chem. 2011, 399, 2407–2411. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Pillay, J.; Ozoemena, K.I. Probing the electrochemical behaviour of SWCNT-cobalt nanoparticles and their electrocatalytic activities towards the detection of nitrite at acidic and physiological pH conditions. Electrochim. Acta 2010, 14, 4319–4327. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.S.; Yin, Z.Z.; Chen, X.H.; Wang, X.Q.; Wu, D.T.; Kong, Y. Electropolymerized melamine for simultaneous determination of nitrite and tartrazine. Food Chem. 2020, 333, 127532. [Google Scholar] [CrossRef]
- Weni, A.; Wulan, T.W.; Mohamad, R.; Budi, R.P. Electrochemical sensor based on graphene oxide/PEDOT:PSS composite modified glassy carbon electrode for environmental nitrite detection. Int. J. Electrochem. Sci. 2023, 18, 100034. [Google Scholar]
- Sarvajith, M.S.; Mruthyunjayachari, C.S.; Fasiulla, K.; Sheela, T.; Harish Makri, N.K. Highly sensitive and selective detection of nitrite by polyaniline linked tetra amino cobalt (II) phthalocyanine surface functionalized ZnO hybrid electrocatalyst. Surf. Interfaces 2023, 36, 102565. [Google Scholar]
- Ding, F.; Zhang, G.; Chen, C.P.; Jiang, S.J.; Tang, H.; Tan, L.; Ma, M. A metal-organic gel-carbon nanotube nanocomposite for electrochemical detection of nitrite. ACS Appl. Electron. Mater. 2021, 3, 761–768. [Google Scholar] [CrossRef]
- Lavanya, A.L.; Kumari, K.G.B.; Prasad, K.R.S.; Brahman, P.K. Development of pen–type portable electrochemical sensor based on Au–W bimetallic nanoparticles decorated graphene-chitosan nanocomposite film for the detection of nitrite in water, milk and fruit juices. Electroanalysis 2021, 33, 1096–1106. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhu, H.Q.; Li, S.N.; Zhai, Q.G.; Chen, Y.; Jiang, Y.C. Ionic liquid modified chloroperoxidase is immobilized on gold nanoparticles-reduced graphene oxide nanocomposites for efficient sensoring of nitrite by electroenzymatic catalysis. Sens. Actuators B Chem. 2022, 371, 132592. [Google Scholar] [CrossRef]










| Electrode | Linear Range (μM) | Sensitivity (μA/μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| Facile synthesis of graphene oxide–silver nanocomposite-modified glassy carbon electrode | 10–180 | / | 2.1 | [41] |
| Cytochrome c-type nitrite redutase/Nafion/methylviologen-modified glassy carbon electrode | 75–800 | / | 60 | [42] |
| Silver particle–ionic liquid-modified carbon paste electrode | 50–1000 | / | 3 | [43] |
| Reduced graphene/Pd nanocomposite-modified glassy carbon electrode | 0.536–108 | 0.06 | 0.015 | [44] |
| Nafion/single-layer graphene nanoplatelet-tetrasodium 1,3,6,8-pyrenetetrasulfonic acid-myoglobin-modified glassy carbon electrode | 50–2500 | 0.0006 | 10 | [45] |
| Reduced graphene oxide-modified screen-printed electrode | 20–100 100–1000 | 0.015 0.007 | 0.83 | [46] |
| Platinum nanoparticles/4-benzenamine (2-aminoethyl)/3-mercaptopropionic acid-modified gold electrode | 10–1000 | 0.02 | 5 | [47] |
| Cobalt nanoparticles/single-walled carbon nanotube-modified edge plane pyrolytic graphite electrode | 32–200 | 0.25 | 5.61 | [48] |
| Poly-(melamine) modified glassy carbon electrode | 10–400 | 0.01 | 1.86 | [49] |
| Graphene oxide/poly (3,4-ethylenedioxythiophene) poly-(styrenesulfonate) modified glassy carbon electrode | 1–50 50–200 | 0.026 0.016 | 0.5 | [50] |
| Polyaniline-linked tetra amino cobalt (II) phthalocyanine surface-functionalized ZnO hybrid nanomaterial-modified glassy carbon electrode | 50–500 | 0.45 | 0.021 | [51] |
| Metal-organic gel-multiwalled carbon nanotube nanocomposite-modified glassy carbon electrode | 0.3–100 | 0.037 | 0.086 | [52] |
| Gold-Tungsten bimetallic nanoparticle-decorated graphene-chitosan-modified pencil graphite electrode | 10–250 | 0.9 | 0.12 | [53] |
| Chloroperoxidase-ionic liquid/reduced graphene oxide-gold nanoparticle-modified glassy carbon electrode | 0.5–300 | 0.05 | 0.049 | [54] |
| Poly-(tea polyphenols) silver nanoparticle-modified pencil graphite electrode | 0.02–860 860–1160 | 0.05 0.14 | 0.004 | This work |
| Samples | Nitrite Found (μM ± RSD, n = 5) | |
|---|---|---|
| The Present Method | Spectrophotometry | |
| Duck feet | 0.183 ± 0.07 | 0.178 ± 0.06 |
| Sausage | 2.263 ± 0.23 | 2.257 ± 0.31 |
| Bacon | 0.257 ± 0.05 | 0.250 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, D.; Zhu, C.; Liu, X.; Wang, Y.; Lu, Y.; Zheng, D.; Fu, B. Fabrication of a Disposable Amperometric Sensor for the Determination of Nitrite in Food. Micromachines 2023, 14, 687. https://doi.org/10.3390/mi14030687
Liu C, Chen D, Zhu C, Liu X, Wang Y, Lu Y, Zheng D, Fu B. Fabrication of a Disposable Amperometric Sensor for the Determination of Nitrite in Food. Micromachines. 2023; 14(3):687. https://doi.org/10.3390/mi14030687
Chicago/Turabian StyleLiu, Chao, Daoming Chen, Chunnan Zhu, Xiaojun Liu, Yu Wang, Yuepeng Lu, Dongyun Zheng, and Baorong Fu. 2023. "Fabrication of a Disposable Amperometric Sensor for the Determination of Nitrite in Food" Micromachines 14, no. 3: 687. https://doi.org/10.3390/mi14030687
APA StyleLiu, C., Chen, D., Zhu, C., Liu, X., Wang, Y., Lu, Y., Zheng, D., & Fu, B. (2023). Fabrication of a Disposable Amperometric Sensor for the Determination of Nitrite in Food. Micromachines, 14(3), 687. https://doi.org/10.3390/mi14030687