Hybrid Microfluidic Platform for Multifactorial Analysis Based on Electrical Impedance, Refractometry, Optical Absorption and Fluorescence
Abstract
:1. Introduction
2. Materials and Methods
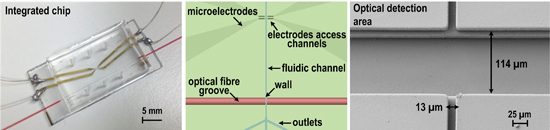
2.1. Microfluidic Chip Fabrication and Characterization
2.2. Microfluidic Setup
2.3. Impedance Analysis Setup
2.4. Optical Setup
2.5. Sample Preparation
3. Results
3.1. Development of Microfluidic Chip
3.2. Electrical Analysis Results
3.3. Optical Analysis Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abgrall, P.; Gue, A. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in microfluidics for environmental analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gai, H.; Li, Y.; Yeung, E.S. Optical Detection Systems on Microfluidic Chips, in Microfluidics; Springer-Verlag: Berlin, Germany, 2011; pp. 171–201. [Google Scholar]
- Zhao, L.; Wu, T.; Lefèvre, J.-P.; Leray, I.; Delaire, A.J. Fluorimetric lead detection in a microfluidic device. Lab Chip 2009, 9, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lin, Y.; Wang, J. Monitoring environmental pollutants by microchip capillary electrophoresis with electrochemical detection. Talanta 2006, 68, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Helseth, L.E. Simultaneous measurements of absorption spectrum and refractive index in a microfluidic system. Opt. Express 2012, 20, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Barat, D.; Spencer, D.; Benazzi, G.; Mowlemb, M.C.; Morgan, H. Simultaneous high speed optical and impedance analysis of single particles with a microfluidic cytometer. Lab Chip 2012, 12, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Caselli, F.; Shaker, M.; Colella, L. Modeling, simulation, and performance evaluation of a novel microfluidic impedance cytometer for morphology-based cell discrimination. J. Microelectromech. Syst. 2014, 23, 785–794. [Google Scholar] [CrossRef]
- Cheung, K.C.; Di Berardino, M.; Schade-Kampmann, G.; Hebeisen, M.; Pierzchalski, A.; Bocsi, J.; Mittag, A.; Tárnok, A. Microfluidic impedance-based flow cytometry. Cytometry A 2010, 77, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Kim, K.H.; Kim, H.C.; Chung, T.D. A portable microfluidic flow cytometer based on simultaneous detection of impedance and fluorescence. Biosens. Bioelectron. 2010, 25, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Su, H.W.; Prieto, J.; Voldman, J. Automated high-throughput characterization of cells using multimodal electrical and optical cytometry (MultiMEOC). In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2012), Okinawa, Japan, 28 October–1 November 2012.
- Kummrow, A.; Theisen, J.; Frankowski, M.; Tuchscheerer, A.; Yildirim, H.; Brattke, K.; Schmidt, M.; Neukammer, J. Microfluidic structures for flow cytometric analysis of hydrodynamically focussed blood cells fabricated by ultraprecision micromachining. Lab Chip 2009, 9, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Borisova, E.; Vladimirov, B.; Ivanova, R.; Avramov, L. Light-Induced Fluorescence Techniques for Gastrointestinal Tumour Detection; Intech: Rijeka, Croatia, 2011. [Google Scholar]
- Chin, L.; Liu, A.Q.; Lim, C.S.; Yap, P.H. Multiphase flow manipulation for continuous refractive index analysis of single living cell. In Proceedings of the Eleventh International Conference on Miniaturized Systems for Chemistry and Life Sciences, Paris, France, 7–11 October 2007.
- Chin, L.; Liu, A.Q.; Lim, C.S.; Zhang, X.M.; Ng, J.H.; Hao, J.Z.; Takahashi, S. Differential single living cell refractometry using grating resonant cavity with optical trap. Appl. Phys. Lett. 2007, 91, 243901. [Google Scholar] [CrossRef]
- Shao, H.; Kumar, D.; Lear, K.L. Single-cell detection using optofluidic intracavity spectroscopy. IEEE Sens. J. 2006, 6, 1543–1550. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.M.; Liu, A.Q.; Lim, C.S.; Yap, P.H.; Hosseini, H.M.M. Refractive index measurement of single living cells using on-chip Fabry-Pérot cavity. Appl. Phys. Lett. 2006, 89, 203901. [Google Scholar] [CrossRef]
- Braschler, T.; Metref, L.; Zvitov–Marabi, R.; van Lintel, H.; Demierre, N.; Theytaza, J.; Renauda, P. A simple pneumatic setup for driving microfluidics. Lab Chip 2007, 7, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.M.; Nogueira, N.; Silva, T.; Braschler, T.; Demierre, N.; Renaud, P.; Oliva, A.G. Dielectrophoretic sorting on a microfabricated flow cytometer: Label free separation of Babesia bovis infected erythrocytes. Bioelectrochemistry 2008, 73, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Sandison, M.E.; Green, N.G. On-chip high-speed sorting of micron-sized particles for high-throughput analysis. IEE Proc. Nanobiotechnol. 2005, 152, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Holmes, D.; Green, N.G. High speed simultaneous single particle impedance and fluorescence analysis on a chip. Curr. Appl. Phys. 2006, 6, 367–370. [Google Scholar] [CrossRef]
- Morgan, H.; Sun, T.; Holmes, D.; Gawad, S.; Green, N.G. Single cell dielectric spectroscopy. J. Phys. D Appl. Phys. 2006, 40, 61. [Google Scholar] [CrossRef]
- Küttel, C.; Nascimento, E.; Demierre, N.; Silve, T.; Braschler, T.; Renaud, P.; Oliva, A.G. Label-free detection of Babesia bovis infected red blood cells using impedance spectroscopy on a microfabricated flow cytometer. Acta trop. 2007, 102, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, F.; Gaur, A.; Sun, Y. Processing dependent behavior of soft imprint lithography on the 1–10-nm scale. IEEE Trans. Nanotechnol. 2006, 5, 301–308. [Google Scholar]
- Del Campo, A.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81. [Google Scholar] [CrossRef]
- Bernacka-Wojcik, I.; Lopes, P.; Vaz, A.C.; Veigasa, B.; Wojcik, P.J.; Simões, P.; Barata, D.; Fortunato, E.; Baptista, P.V.; Águas, H.; et al. Bio-microfluidic platform for gold nanoprobe based DNA detection—Application to Mycobacterium tuberculosis. Biosens. Bioelectron. 2013, 48, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.P.; Freeman, D.M.; Voldman, J. Plastic masters—Rigid templates for soft lithography. Lab Chip 2009, 9, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Adrega, T.; Lacour, S. Stretchable gold conductors embedded in PDMS and patterned by photolithography: Fabrication and electromechanical characterization. J. Micromech. Microeng. 2010, 20, 055025. [Google Scholar] [CrossRef]
- Guo, L.; DeWeerth, S.P. An Effective Lift-Off Method for Patterning High-Density Gold Interconnects on an Elastomeric Substrate. Small 2010, 6, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Marelli, M.; Divitini, G.; Collini, C.; Ravagnan, L.; Corbelli, G.; Ghisleri, C.; Gianfelice, A.; Lenardi, C.; Lorenzelli, L.; Milani, P. Flexible and biocompatible microelectrode arrays fabricated by supersonic cluster beam deposition on SU-8. J. Micromech. Microeng. 2011, 21, 045013. [Google Scholar] [CrossRef]
- Ge, J.; Kivilahti, J. Effects of surface treatments on the adhesion of Cu and Cr/Cu metallizations to a multifunctional photoresist. J. Appl. Phys. 2002, 92, 3007–3015. [Google Scholar] [CrossRef]
- Vilares, R.; Hunter, C.; Ugarte, I.; Aranburu, I.; Berganzo, J.; Elizalde, J.; Fernandez, L.J. Fabrication and testing of a SU-8 thermal flow sensor. Sens. Actuators B 2010, 147, 411–417. [Google Scholar] [CrossRef]
- Agirregabiria, M.; Blanco, F.J.; Berganzo, J.; Arroyo, M.T.; Fullaondo, A.; Mayora, K.; Ruano-López, J.M. Fabrication of SU-8 multilayer microstructures based on successive CMOS compatible adhesive bonding and releasing steps. Lab Chip 2005, 5, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.; Fernández, L.J.; Agirregabiria, M.; Ibañez1, N.; Aurrekoetxea, J.; Blanco, F.J. Novel all-polymer microfluidic devices monolithically integrated within metallic electrodes for SDS-CGE of proteins. J. Micromech. Microeng. 2007, 17, 1289. [Google Scholar] [CrossRef]
- Tillak, J.B.; Bernacka-Wojcik, I.; Barata, D.; Jorge, P.A.S.; Águas, H.; Oliva, A.G. Towards single cell spectroscopy and refractometry in microfluidic chip platforms. In Proceedings of the International Conference on Applications of Optics and Photonics, Braga, Portugal, 3 May 2011; Costa, M.F., Ed.; pp. 800112–800118.
- Bang, H.; Yun, H.; Lee, W.G.; Park, J.; Lee, J.; Chung, S.; Cho, K.; Chung, C.; Han, D.C.; Chang, J.K. Expansion channel for microchip flow cytometers. Lab chip 2006, 6, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.S. Shrinkage ratio of PDMS and its alignment method for the wafer level process. Microsyst. Technol. 2008, 14, 205–208. [Google Scholar] [CrossRef]
- Demierre, N.; Braschler, T.; Linderholm, P.; Seger, U.; van Lintel, H.; Renaud, P. Characterization and optimization of liquid electrodes for lateral dielectrophoresis. Lab Chip 2007, 7, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Martinho, I.; Tillak, J.B.; Bernacka-Wojcik, I.; Barata, D.; Jorge, P.A.S.; Águas, H.; Oliva, A.G. Microfluidic chip for spectroscopic and refractometric analysis. In Proceedings of the OFS2012 22nd International Conference on Optical Fibre Sensors, Braga, Portugal, 17 October 2012; pp. 84211–84214.
- Gouveia, C.; Zibaiic, M.; Latific, H.; Marquesd, M.J.B.; Baptistaa, J.M.; Jorge, P.A.S. High resolution temperature independent refractive index measurement using differential white light interferometry. Sens. Actuators B 2013, 188, 1212–1217. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.M.; Bernacka-Wojcik, I.; Ribeiro, R.S.R.; Lobato, M.T.; Fortunato, E.; Martins, R.; Igreja, R.; Jorge, P.A.S.; Águas, H.; Oliva, A.M.G. Hybrid Microfluidic Platform for Multifactorial Analysis Based on Electrical Impedance, Refractometry, Optical Absorption and Fluorescence. Micromachines 2016, 7, 181. https://doi.org/10.3390/mi7100181
Pereira FM, Bernacka-Wojcik I, Ribeiro RSR, Lobato MT, Fortunato E, Martins R, Igreja R, Jorge PAS, Águas H, Oliva AMG. Hybrid Microfluidic Platform for Multifactorial Analysis Based on Electrical Impedance, Refractometry, Optical Absorption and Fluorescence. Micromachines. 2016; 7(10):181. https://doi.org/10.3390/mi7100181
Chicago/Turabian StylePereira, Fábio M., Iwona Bernacka-Wojcik, Rita S. Rodrigues Ribeiro, Maria Teresa Lobato, Elvira Fortunato, Rodrigo Martins, Rui Igreja, Pedro A. S. Jorge, Hugo Águas, and Abel Martin Gonzalez Oliva. 2016. "Hybrid Microfluidic Platform for Multifactorial Analysis Based on Electrical Impedance, Refractometry, Optical Absorption and Fluorescence" Micromachines 7, no. 10: 181. https://doi.org/10.3390/mi7100181
APA StylePereira, F. M., Bernacka-Wojcik, I., Ribeiro, R. S. R., Lobato, M. T., Fortunato, E., Martins, R., Igreja, R., Jorge, P. A. S., Águas, H., & Oliva, A. M. G. (2016). Hybrid Microfluidic Platform for Multifactorial Analysis Based on Electrical Impedance, Refractometry, Optical Absorption and Fluorescence. Micromachines, 7(10), 181. https://doi.org/10.3390/mi7100181