1. Introduction
A low flow rate, low power, biocompatible micropump capable of periodic flow is critical for drug delivery to small spaces and in small rodent models, such as with ocular and cochlear infusions, and fluid sensitive areas which can be damaged by over perfusion, such as with neural tissue [
1]. Small pumps capable of pumping small volumes of fluid are also needed in a number of applications including portable diagnostics, particle and cell sorting, and cytometry. Resistance to backflow and fluid traveling from target areas is also a requirement for these applications, as studies have shown that backflow from the biological systems into the pump is problematic for infusion rates below 500 nL/min [
2,
3].
Pumps for ocular infusions include both research and commercial devices. Research devices used for rabbits have been designed to pump up to 1.5 µL/min [
4], 5 µL/min to 20 µL/min [
5], and 7.0 µL/min in enucleated porcine eyes [
6]. Commercial devices include the ALZET pump (ALZET Osmotic Pumps, Cupertino, CA, USA), which has been used for transscleral delivery to choroid and retina of rabbits at 133 nL/min [
7]. Pump rates for cochlear infusions in small rodent models require even lower flow rates than are currently available due to smaller fluid volume and pressure sensitive structures. In the mouse model, infusions were performed at 17 nL/min [
8], 16 nL/min to 32 nL/min [
9], and up to 50 nL/min with posterior semicircular canal canalostomy [
10].
Point of care diagnostic systems often rely on small sample sizes that necessitate low flow rate pumps [
11]. These types of devices have been designed with flow rates of up to 100 nL/min [
12,
13,
14]. For example, micro total analysis systems (µTAS) perform sample manipulation on small fluid volumes less than 1 nL [
15], including sample delivery to perform mixing, reaction, and analysis.
In drug delivery applications diffusion often plays a role in delivery, but can also result in undesired diffusion mediated transport during periods when the pump is inactive. Therefore, a means of blocking the flow to isolate the drug reservoir from the biological system is needed for implantable systems, which can be difficult in these power-constrained applications. While none of the research pumps presented above achieve blocked flow, commercial products such as the SynchroMed II from Medtronic (Minneapolis, MN, USA) pump as low as 0.6 µL/min and do provide flow blockage, but only by powering the pump in the off position. The iPRECIO pumps (ALZET Osmotic Pumps, Cupertino, CA, USA) are essentially miniaturized versions of a standard peristaltic pump using pins to compress tubing in place of the standard roller mechanism and provide flow rates down to 60 µL/min. While both of these are programmable and provide blocked flow, the minimum flow rates are much larger than those required for implantable small rodent and μTAS systems. Additionally, they require power to maintain the blocked-flow condition. There is a need for ultra-low flow rate implantable pumps that provide the flexibility of the iPRECIO pump, with the added benefit of blocked flow in the unpowered state.
Valves can be active such as a diaphragm actuated with external force, or passive as in the case of a flap check valve where pressure of the pumped fluid opens and closes the valve. This type of valve requires a minimum volume to deform the valve flap sufficiently before fluid begins to flow. Check valves have been reported with dimensions ranging from 70,000 μm
2 [
16] to 2 mm
2 [
17], which are too large for low flow rate micropumps. Passive valves also include fixed geometry valves, which rely on the fluid mechanics within the channel to favor flow in one direction over another, resulting in flow rectification. However, in the laminar flow regime, hydrodynamics must be considered when evaluating the utility of passive valves [
18,
19,
20]. These passive valves rely on the hydrodynamics of the system to produce a valve effect, so the Reynolds number (
Re), the ratio of the inertial to viscous forces, defines valve performance. Systems with low
Re require careful consideration in the design phase to ensure sufficient flow rectification to enable pumping. The critical number below which flow is completely laminar is
Re = 15; below this value, valves relying on pressure loss coefficients (e.g., fixed geometry valves) have been shown not to function [
21], and check valves exhibit efficiencies below 12% [
22].
Figure 1 shows a graph of
Re compared to volumetric flow rates for various channel cross sectional areas (
ACS). For microfluidic cross-sectional areas that can be easily fabricated (e.g.,
ACS > 10 μm
2), flows remain completely laminar for flow rates below 3000 nL/min, making passive valves impractical. This remains true even for peristaltic pumps with pulsatile flow, as long as the instantaneous flow rate associated with displacement actuation remains below this critical value. Hence, in this work, active valves were pursued.
There are numerous actuation mechanisms that can be used with active valves including electrostatic, piezoelectric, electromagnetic, electro-osmotic, electrolysis, magneto-hydrodynamic, thermodynamic, continuous electro-wetting, shape memory material, and phase-change material [
23,
24,
25]. For low power consumption in a programmable micropump, it is important to use normally closed valves to restrict flow without expending energy. Normally closed active valves can be achieved with shape memory alloys, however these generally require relatively high temperatures to deform (e.g., 75
°C [
26]) that may be incompatible with the biological system or degrade the drug being pumped. An alternative is phase-change materials that expand when they solidify, and shrink during melting. Gallium is a suitable material for this application as it has a melting point sufficiently low to prevent damage to the biological system, and it undergoes orthorhombic crystallization as it cools causing the material to expand as the crystals are formed. In this paper we describe a gallium actuator in a fully integrated micropump with the underlying chamber-based peristaltic pump providing the active valves required for zero-power blocked flow. The pump was created with a hybrid Micro Electro Mechanical System (MEMS) approach including the integration of biocompatible membranes and channels [
27] with the Ga actuator using additive manufacturing, direct write techniques. The performance of the fully integrated pump was characterized including chamber volumes, pump rates, and pump efficiency.
2. Design
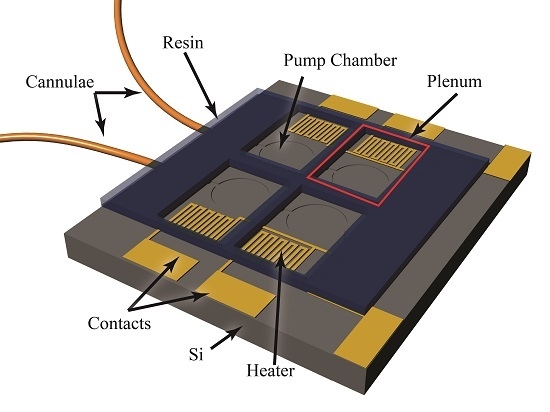
The foundation of the peristaltic pump is a silicon-based MEMS device, which includes fluidic channels, in-plane interconnects, pump chambers, and an etched aluminum film creating heaters and contact pads as illustrated in
Figure 2. Four phase-change actuators were used in this pump to directionally motivate the fluid peristaltically by transferring momentum to the underlying Tetraethyl Orthosilicate (TEOS)/Parylene-C diaphragms. Parylene-C was chosen as it is a non-reacting, non-water-adsorbing, biocompatible material that can be deposited in a pseudo-conformal process [
28].
Parylene-C has been shown to be biocompatible with
in vitro studies including live/dead staining and cell viability [
29], and
in vivo tests for inflammation [
30] and tissue morphologic changes [
31,
32]. Material structural stability when implanted has been confirmed with long-term
in vivo studies [
33]. The demonstrated biocompatibility, biostability, and the ability to encapsulate implant devices [
34] make Parylene-C an ideal coating for implantation. The presented device used Parylene in four ways, first to form the pump chamber diaphragms, second to provide a biocompatible flow path, third to capture small diameter tubing for fluid delivery, and fourth to encapsulate the entire device in a stable, biocompatible coating.
Aluminum resisters were used to transfer actuation energy in the form of heat to the gallium, which resides in plena formed via a direct-write method over the pump chambers. The rigid plena provide a constant volume above each diaphragm, such that expansion and contraction of the gallium results in actuation of the diaphragm. As the gallium is heated to its melting point, it loses its orthorhombic crystal structure and collapses, resulting in a reduction in volume and upward movement of the flexible diaphragm pulling fluid into the underlying pump chamber. As the gallium cools and freezes, expansion forces the diaphragm down, pushing fluid out of the chamber and effectively closing the flow path. The actuation mechanism and pump structure are illustrated in
Figure 3.
The compression ratio of the pump chambers needs to be great enough to overcome back pressure from the downstream system (e.g., biological system). For flow rates appropriate for implantable systems in small rodent models, peristaltic pump chambers need to be tens of microns deep and hundreds of microns in diameter to provide sufficient compression ratio (diaphragm displacement volume to total chamber volume) to deliver fluid against back pressure [
23]. With the current fabrication process, chambers of up to 1000 µm can easily be fabricated. Channels, the same depth as the chambers, can easily be fabricated as narrow as 10 µm and as wide as the pump chambers. For the current work, channels 100 µm wide have been used providing a channel cross-sectional area of 1500 μm
2 and completely laminar flow for flow rates up to 60 μL/min. Chamber and membrane designs ranged from 500 to 800 µm diameter, 15 µm deep with 3 to 4.5 µm of deposited Parylene C (PCPX) deposited over 1 μm of TEOS.
The maximum back-pressure against which the gallium actuators can work was estimated from the bulk modulus of Ga (56.9 GPa). Gallium expands 3.1% when it solidifies, providing a theoretical, maximum pressure of 1.76 GPa to the diaphragm and underlying fluid. This is more than ten times the expansion pressure provided by a paraffin wax actuator with a bulk modulus of 1.66 GPa and expansion of 10% yielding a theoretical pressure of 167 MPa. Measured actuation pressures for paraffin-based designs yielded 27% [
35] of the theoretical prediction, suggesting a gallium-based device can provide at least 480 MPa of pressure for robust actuation and pumping against back-pressure.
3. Fabrication
Figure 4 shows a cross-sectional drawing of the pump fabrication process, which builds on integration technologies from [
36]. Wafers were RCA cleaned and 0.5 μm of plasma-enhanced chemical vapor deposition (PECVD) Tetraethyl Orthosilicate (TEOS) SiO
2 was deposited (Applied Materials P5000, 290 W, 68 s). The TEOS was patterned via a plasma etch (Drytek Quad, Ar: 35 sccm, CHF
3: 65 sccm, O
2: 5 sccm, 200 W, 70 mtorr) with a grid of 1-µm openings to define the fluid channel, interconnect, and diaphragm regions. A 0.3-µm film of aluminum was evaporated onto the surface in a CHA flash evaporator system. A contact mask was manually aligned to the patterned TEOS with exposure performed on a contact aligner (MA-150, Karl Suss America, Waterbury, VT, USA). Heaters and contacts were patterned in the aluminum with wet etching, Aluminum Etch 16-1-1-2 (Fujifilm Corporation, Tokyo, Japan).
The microfluidic channels, pump chambers, and interconnects to accept micro capillary tubing were formed with a two-step process that creates both shallow flow channels and deeper channels for the microfluidic interconnect. A film of photoresist was patterned to cover the shallow channel and chamber regions from an initial XeF
2 etch process. The photoresist was then removed in an O
2 plasma followed by a second XeF
2 etch to form the shallow flow channels and pump chambers. This two-step process achieves different depths for the flow path (deep interconnects of 145 µm; shallow channels of 15 µm). The diaphragms were formed over the chambers by filling in the grid of 1-µm patterned openings in the TEOS film with a room temperature deposition of 3 µm of Parylene-C using a custom designed deposition tool. The thickness of the Parylene-C deposition was controlled by the amount of dimer placed in the evaporator at the start of the process, with one gram of dimer yielding 0.8 µm of polymer at a deposition pressure of 30 mtorr. This deposition simultaneously coats the microfluidic channels with biocompatible parylene while creating the deformable pump membranes [
36]. Polyimide tape was applied to the resistor contact pads prior to deposition to keep these regions free of Parylene-C.
Microfluidic interconnects to polyimide microcapillary tubing were created as described in [
37]. Briefly, a 0.17 mm cover glass (18 mm × 18 mm) was adhered to the chip along the interconnect edge to prevent resin from flowing into the microchannels during the direct write step and to provide a clean opening for the insertion of the capillary tubing. The wafer was diced with a wafer saw (K&S 780, Kulicke & Soffa Pte. Ltd., Singapore, Singapore) to expose the microchannel interface. Using a direct write system (nScrypt, Orlando, FL, USA) the actuator plenums were printed directly on top of the diaphragm device with UV curable resin to define the gallium wells. The resin was dispensed from a pneumatically controlled syringe (20.1 psi) through a 75 µm ID tip to control the width and height of lines defined in a tool path text file. The device was held onto the tool’s platen with vacuum and moved along the path at 5 mm/s. The resin gel (F-134, Art Clay World, Jacksonville, TX, USA) was cured under a 365 nm UV light for 10 min. Small diameter capillary tubing (140 µm OD) was inserted into the interface openings and sealed with a thick (40 µm) deposition of Parylene-C as described in [
37]. Polyimide tape was applied the tops of the plena prior to deposition to keep these free of Parylene-C.
To facilitate the precise measurement of the minute volume of Ga needed (412 nL of solidified Ga), lengths of long flat Ga ‘ingots’ were formed using a micro stamp-mold process using a polydimethylsiloxane (PDMS) mold. As shown in
Appendix A,
Figure A1, a positive mold was created using 105-μm thick SU-8 3010 photoresist (MicroChem Corp. Westborough, MA, USA) on a 100-mm silicon wafer. This positive mold was then used to cast the negative mold in PDMS, which had been processed under vacuum for 60 min to remove bubbles, followed by curing at 70
°C for 60 min. Individual stamps were razor cut with vent holes (1/2 mm OD) punched using a metal cylinder. Gallium was placed on a clean silicon wafer and melted on a hot plate at 100
°C. The stamp was pressed down on top of the molten gallium and held with a small weight. The wafer was then placed on an ice pack to speed cooling and solidification of the gallium micro-ingots, which were easily removed from the pliable PDMS mold. The resulting micro-printed ingots were 0.8 mm wide, 90 µm thick, and 20 mm long, providing a Ga volume of 72 nL per 1 mm length. Ingots were cut to a length of 5.7 mm with a razor blade under a 40× microscope, providing 412 ± 7.2 nL of Ga for placement in the pump actuation plenums.
The capillary tubing was connected with Nanotight fittings (Upchurch, Scientific, Oak Harbor, WA, USA) to vacuum of less than 240 torr (providing a trans-diaphragm pressure of >10 psi) to hold the diaphragms in the down (closed) position in order to deposit the gallium. This ensures the diaphragms are actuated to the closed position by the solidified metal. Gallium micro ingots were placed in the plena and melted on a hot plate at 100 °C, such that they would conform to the shape of the actuated diaphragms while the pumps were under vacuum.
The device was removed from the hotplate and as the metal cooled it expanded and solidified, keeping the diaphragms pushed down as the vacuum was released. A second glass cover slip was coated with UV curable resin and affixed to the device to cover the actuation plena and seal in the gallium, providing a rigid cap for the actuation chambers. This resin was cured for 10 min under a 365 nm wavelength UV light.
5. Results and Discussion
As shown in
Figure 6, a TEOS grid is formed over the channels and chambers etched into the silicon wafer forming the peristaltic pump structure; the flexible PCPX, filled in the grid and formed a contiguous diaphragm. A thin film of Parylene-C was seen to coat the channels and chambers below the diaphragms [
23], providing a biocompatible flow path through the pump.
Figure 7 shows the device prior to Galium placement. The underlying microfluidics and heaters were successfully formed using MEMS techniques with the printed epoxy defining the plena above. The small diameter capillary tubing (140 µm OD) was captured in place via parylene deposition as detailed in [
37].
The diaphragm deflection tests on devices with chamber diameters of 500 μm, a depth of 15 μm, and a diaphragm of 1 μm TEOS and 4.5 μm PCPX showed that the diaphragms were fully deflecting to contact the floor of the etched chamber below. Measurement of the required deflection pressure of 3 psi shows modest agreement with the calculated value of 3.3 psi as shown in
Appendix A. To broaden the potential actuation mechanisms for these diaphragm membranes, additional devices were fabricated with a larger diameter chamber (800 μm) and slightly thinner PCPX (3 μm) to reduce the required actuation force. While the gallium provides more than adequate actuation pressure, the more compliant diaphragms may enable adaptation to other actuation mechanisms with lower force capabilities such as electro-osmosis, electrolysis, and piezoelectric [
37].
In the long-term testing, seven diaphragms (500 μm diameter, 1 μm TEOS, and 4.5 μm PCPX) were actuated for 307 days with over 26 million (26,524,800) actuations with no failures. This is the equivalent of over 185 million diaphragm actuations without a single failure. These results suggest the composite TEOS/Parylene membranes provide a robust interface between the actuator and the pumped fluid.
Pump actuation tests on devices with chambers 800 μm diameter, 15 μm deep reveal a consistent volume pumped per cycle of 11.1 ± 0.35 nL as shown in
Figure 8. The pump fabrication process results in a volume pumped per cycle that is dependent on the gallium volume and associated expansion. The pump membrane is able to deflect upward and downward, resulting in the capability for greater volumes than those defined by the chamber depth and diameter alone. The average pumped volume is 0.9 nL (7.5%) lower than designed which may be due to three sources of error. The pumped fluid is controlled by the dimensional change of the gallium, which is directly correlated to the Ga volume. The cutting error of the gallium was estimated to be ±7.2 nL; this would account for a pumped volume error of only ±0.01 nL. Another potential source of error is over heating of the gallium past the melting point due to open-loop heater control. COMSOL simulations of the pump structure suggests the Ga will reach temperatures of 306 K, three degrees higher than the melting point of pure Ga. The melted gallium will re-expand after the initial melted state is reached. The 0.9 nL reduction in pumped volume corresponds to a reduction in Ga density of 6.075 g/cm
3 and a temperature overshoot of 20
°C, much higher than estimated with COMSOL so this is unlikely to be the source of error. The last potential source of error is from the integration process when the molten gallium is placed over the diaphragms as they are being held down with a vacuum. Deflection differences as small as 1.8 µm could explain the 0.9 nL volume error. For the future, positive pressure deflection during gallium integration may provide more robust membrane deflection and reduce this relatively small volume error.
Flow rates were measured as described above for actuation frequencies from 0.02 to 0.083 Hz as shown in
Figure 9, with pump rates between 18 and 104 nL/min achieved at an activation power of 10.1 mW and a Gallium temperature of 306 K. This four chamber peristaltic pump delivers two pump chamber volumes (e.g., 22.2 nL) every period, yielding an efficiency of 11 mJ/nL. These results compare favorably to phase change actuation micropumps operating in the laminar flow regime where efficiencies of 48 mJ/nL [
38] have been reported. However, this Ga-based actuator offers additional efficiency benefits compared to traditional normally open micropumps for complex pump regiments and very low flow rate applications. Both require the pump to be off or paused for periods of time; the Ga-actuator closes off the flow channel when power is removed, eliminating the need to power an active valve. While the lowest pump rate presented was 18 nL/min, lower rates can be achieved by cyclic pausing of the pump with all chambers closed. Achieving pump rates higher than 104 nL/min can be achieved through increasing the actuation current to shorten melt times, or by increasing the size of the pump chambers and associated Ga.
The melting point of pure Gallium is 303 K (30
°C) and required a temperature rise of 10
°C from room temperature of 293 K for our pump testing. There are two main issues associated with this temperature range: a large shift in temperature requires power, and the melting point is below body temperature (37
°C). The melting point needs to be optimized for use in implantable systems using a gallium-indium (Ga-In) alloy; An alloy of 23% atomic weight In will provide an increase in the melting point from 30 to 40
°C [
39]. Since the melting point of indium is relatively low (156
°C) and the boiling point of Ga is over 2200
°C, the alloy can be formed without significant loss of either metal. An alloy with a transition temperature tuned to be just a few degrees above body temperature will reduce power requirements for heating and improve efficiency. It is expected that reducing the required temperature rise from 10 to 3
°C could yield pump efficiencies of 9.2 mJ/nL.
COMSOL simulations of the pump structure reveals overshoot in target gallium temperature due to the constant application of power even after the gallium had reached its melting temperature. Closed loop control would enable attenuation of power as the temperature approaches the melting point to eliminate this overshoot. The COMSOL model suggests power reductions down to 6.3 mW are possible, providing a theoretical pump efficiency of 6.8 mJ/nL. Closed-loop control would require integration of temperature sensing within the plenum, which could be achieved with thermopiles as described by [
40,
41,
42]. A recent review by Ogden on miniature paraffin phase change actuators, valves and pumps [
43] provides insight into the power and associated flow rates for a range of system designs. The most efficient of the pumps reviewed [
44], was shown to have flow rates as low as 80 nL/min but at an estimated efficiency of 18 mJ/nL, roughly three times less efficient than the Ga pump presented here.
The pump rate is easily controlled via the frequency of the control circuit; this pump demonstrated a large range of pump rates (18 to 104 nL/min). For much lower flow rates, smaller chambers could be used, along with smaller gallium plenums. The chambers used here were 800 µm in diameter and 15 µm deep. Chambers as small as 400 µm in diameter and 5 µm deep to as large as 1500 µm in diameter and 20 µm deep have been successfully fabricated. This range of demonstrated chamber sizes enables scaling of the flow rate from 1.5 to 0.5 μL/min with the described MEMS technology. Multiple flow channels could be employed to magnify the flow rates. For example two pumps in parallel could pump up to 1 µL/min and if the chambers of each pump were arranged linearly, the pumps could share electronics reducing the device’s complexity. To increase the flow rate beyond µL/min flow rates, other chamber and diaphragm technologies could be integrated with the gallium actuators. Molded PDMS chambers and diaphragms, used for thermodynamic actuation, can be fabricated with diaphragm diameters >5 mm [
45].
The pseudo-conformal nature of the Parylene-C deposition process ensures that all flow paths are coated with a non-reacting, non-water-adsorbing, biocompatible material [
31,
37]. This allows the pumping of fluids commonly used for infusions, including pharmaceuticals and biologic agents without altering the fluids or the pump. Even with the biocompatibility of the Parylene-C encapsulation of the device, concerns of potential toxicity leakage of materials must be addressed. The toxicity of Ga, In, and Ga-In alloys in humans is not well studied and the Material Safety Data Sheet for Ga-In only presents limits on inhalation (<0.1 mg/m
3) [
46]. Small animal studies in rats with intratracheal administration of Ga, at doses of 24, 48, and 96 mg/kg, revealed temporary toxicity effects (<18 days) for highest dose only, but that no effects were seen for lower doses [
47]. The oral median lethal dose (LD50) of Ga in mice and rats is more than 15 g/kg, and the threshold for acute effects (TAE) is 7 g/kg [
48]. The oral LD50 of indium is 4.2 g/kg for rats [
49]. The presented micropump designed for small rodents would contain 0.22 mg of Ga and 0.064 mg of In, which for a 10 g mouse is the equivalent of 22 and 6.4 mg/kg respectively. The Ga is two orders of magnitude lower than the TAE and three orders of magnitude lower than the LD50. The In is three orders of magnitude lower than the TAE and four orders of magnitude lower than the LD50. While additional work is required to evaluate potential toxicity of leakage of the Ga-In alloy from a damaged micropump, present literature suggests the small volumes involved may have minimal impact, even in the smallest animal models.