Microfluidic Neurons, a New Way in Neuromorphic Engineering?
Abstract
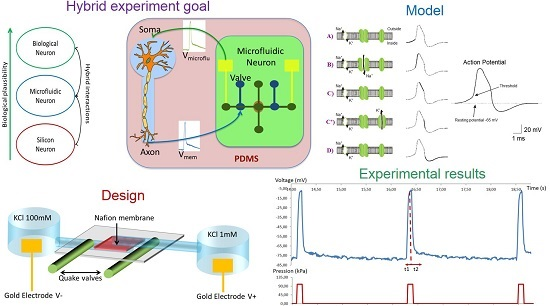
:1. Introduction
2. Novelty and Comparison with the State of the Art in Silicon Neuron
2.1. Silicon Neuron for Hybrid Neural Network
2.2. Microfluidic Neuron for Hybrid Neural Network
3. Design of the Microfluidic Neuron
3.1. Neuron Modelling
3.2. Microfluidic Neuron
3.3. Design Steps
4. Results
5. Discussion
5.1. Hybrid Experiments
5.2. New Design Ideas
5.3. Microfluidic Neuron as a Stimulator
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nicolelis, M.A.L.; Lebedev, M.A. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat. Rev. Neurosci. 2009, 10, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; Smagt, P.V.D.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nat. Methods 2012, 485, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.; Douglas, R. A silicon neuron. Nature 1991, 354, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Le Masson, G.; Renaud-Le Masson, S.; Debay, D.; Bal, T. Feedback inhibition controls spike transfer in hybrid thalamic circuits. Nature 2002, 417, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Ambroise, M.; Levi, T.; Joucla, S.; Yvert, B.; Saighi, S. Real-time biomimetic central pattern generators into FPGA for hybrid experiments. Front. Neurosci. 2013, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Grassia, F.; Buhry, L.; Levi, T.; Tomas, J.; Destexhe, A.; Saighi, S. Tunable neuromimetic integrated system for emulating cortical neuron models. Front. Neurosci. 2011, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whiteside, G. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Indiveri, G.; Linares-Barranco, B.; Hamilton, T.J.; Schaik, A.V.; Etienne-Cummings, R.; Delbruck, T.; Liu, S.C.; Dudek, P.; Häfliger, P.; Renaud, S.; et al. Neuromorphic silicon neuron circuits. Front. Neurosci. 2001, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Mead, C. Neuromorphic electronic systems. Proc. IEEE 1990, 78, 1629–1636. [Google Scholar] [CrossRef]
- Hodgkin, A.; Huxley, A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, G. Synaptic plasticity and spike-based computation in VLSI networks of integrate-and-fire neurons. Neural Inf. Process. Lett. Rev. 2007, 11, 135–146. [Google Scholar]
- Linares-Barranco, B.; Sénchez-Sinencio, E.; Rodríguez-Vázquez, A.; Huertas, J.L. A CMOS implementation of Fitzhugh-Nagumo neuron model. IEEE J. Solid-State Circuits 1991, 26, 956–965. [Google Scholar] [CrossRef]
- Rice, K.L.; Bhuiyan, M.A.; Taha, T.M.; Vutsinas, C.N. FPGA implementation of izhikevich spiking neural networks for character recognition. In Proceedings of 2009 International Conference on Reconfigurable Computing and FPGAs, Cancún, Mexico, 2009; pp. 451–456.
- Schemmel, J.; Brüderle, D.; Grübl, A.; Hock, M.; Meier, K.; Millner, S. A wafer-scale neuromorphic hardware system for large-scale neural modeling. In Proceedings of 2010 IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; pp. 1947–1950.
- Levi, T.; Lewis, N.; Saïghi, S.; Tomas, J.; Bornat, Y.; Renaud, S. Neuromimetic integrated circuits; Artech House: Norwood, MA, USA, 2008; pp. 241–264. [Google Scholar]
- Cassidy, A.; Georgiou, J.; Andreou, A.G. Design of silicon brains in the nano-CMOS era: Spiking neurons, learning synapses and neural architecture optimization. Neural Netw. 2013, 45, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.F.; Cymbalyuk, G.S.; Sorensen, M.E.; Calabrese, R.L.; DeWeerth, S.P. A multi-conductance silicon neuron with biologically matched conductances. IEEE Trans. Biomed. Eng. 2004, 51, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.F.; DeWeerth, S.P. Sensory feedback in a half-center oscillator model. IEEE Trans. Biomed. Eng. 2007, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Sekikawa, M.; Li, J.; Nanami, T.; Aihara, K. Qualitative-modeling-based silicon neurons and their networks. Front. Neurosci. 2016, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Aihara, K. Mathematical-model-based design method of silicon burst neurons. Neurocomputing 2008, 71, 1619–1628. [Google Scholar] [CrossRef]
- Joucla, S.; Ambroise, M.; Levi, T.; Lafon, T.; Chauvet, P.; Saïghi, S.; Bornat, Y.; Lewis, N.; Renaud, S.; Yvert, B. Generation of locomotor-like activity in the isolated rat spinal cord using intraspinal electrical microstimulation driven by a digital neuromorphic CPG. Front. Neurosci. 2016, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, P.; Difato, F.; Massobrio, P.; Breschi, G.L.; Pasquale, V.; Levi, T.; Goldin, M.; Bornat, Y.; Tedesco, M.; Bisio, M.; et al. In vitro large-scale experimental and theoretical studies for the realization of bi-directional brain-prostheses. Front. Neural Circuits 2013, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Biffi, E.; Piraino, F.; Pedrocchi, A.; Fiore, G.B.; Ferrigno, G.; Redaelli, A.; Menegon, A.; Rasponi, M. A microfluidic platform for controlled biochemical stimulation of twin neuronal networks. Biomicrofluidics 2012, 6, 24106–24110. [Google Scholar] [CrossRef] [PubMed]
- Biffi, E.; Menegon, A.; Piraino, F.; Pedrocchi, A.; Fiore, G.B.; Rasponi, M. Validation of long-term primary neuronal cultures and network activity through the integration of reversibly bonded microbioreactors and MEA substrates. Biotechnol. Bioeng. 2012, 109, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.A.; Carnevale, N.; Rozsa, B.; Zecevic, D. Electrical behaviour of dendritic spines as revealed by voltage imaging. Nat. Commun. 2015, 6, 8436. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M. Closing the loop between neurons and neurotechnology. Front. Neurosci. 2010, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Kartalov, E.; Scherer, A.; Weiner, L. Applications of microfluidics for neuronal studies. J. Neurol. Sci. 2007, 252, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.; Cotman, C.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Vahidi, B.; Taylor, A.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, Y.A.; Han, J. Multiplexed proteomic sample preconcentration device using surface-patterned ion-selective membrane. Lab Chip 2008, 8, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.; Trietsch, S.; Vultoa, P.; Hankemeier, T. Elastomeric microvalves as tunable nanochannels for concentration polarization. Lab Chip 2013, 13, 4810–1815. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Jorgolli, M.; Shalek, A.; Yoon, M.H.; Gertner, R.; Park, H. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol. 2012, 7, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal. Chem. 2006, 78, 8169–8174. [Google Scholar] [CrossRef] [PubMed]
















© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levi, T.; Fujii, T. Microfluidic Neurons, a New Way in Neuromorphic Engineering? Micromachines 2016, 7, 146. https://doi.org/10.3390/mi7080146
Levi T, Fujii T. Microfluidic Neurons, a New Way in Neuromorphic Engineering? Micromachines. 2016; 7(8):146. https://doi.org/10.3390/mi7080146
Chicago/Turabian StyleLevi, Timothée, and Teruo Fujii. 2016. "Microfluidic Neurons, a New Way in Neuromorphic Engineering?" Micromachines 7, no. 8: 146. https://doi.org/10.3390/mi7080146
APA StyleLevi, T., & Fujii, T. (2016). Microfluidic Neurons, a New Way in Neuromorphic Engineering? Micromachines, 7(8), 146. https://doi.org/10.3390/mi7080146