1. Introduction
Cells in a sample solution can be largely heterogeneous, consisting of various different sub-populations, even if they originate from the same tumor or cell line. Immunochemistry has been an effective means to investigate sub-populations within a cell solution especially based on specific membrane protein expressions, designated as “cluster of differentiation” (CD). Highly specific immunoaffinity-based assays represent a powerful analysis method, especially with the development of high-throughput cell cytometry. However, the use of biomarkers is still an important limitation due to practical reasons, such as availability of surface markers and corresponding antibodies and heterogeneity between specimens regarding combined marker expressions. Therefore, routine clinical tests for early diagnosis would benefit from cost and time-efficient alternatives.
Cell shape and structural integrity significantly influence many biological processes. Therefore, the physical properties of cells may potentially be used to reflect the state of their health [
1]. This connection between biophysics and disease has been attracting scientific research attention, especially for oncological studies [
2,
3,
4,
5,
6,
7] where diseased cells proliferate “uncontrollably” and disrupt the organization of tissue. When the physical properties of a cell change, the behavior of the cell, e.g., the way they spread, changes as well [
8]. In human cancer cell lines, invasive cells exhibit biophysical properties that are distinct from their noninvasive counterparts with a reduction in stiffness and an increase in metastatic efficiency [
2,
3,
4,
9,
10,
11], especially in the case of circulating tumor cells (CTCs). Therefore, the biophysical properties of cells can be considered as biomarkers to distinguish cells and thereby used as practical label-free indicators for routine clinical examinations targeting early disease diagnosis.
Numerous methods have been used to examine the biophysical properties of single cells: biomechanical assays to probe cell components [
3,
12,
13] or single cell deformation [
10,
14,
15,
16], cytoadherence assays [
8], and microfluidics-based assays for mechanical properties [
5,
17] or for electrical properties [
18]. As the majority of these methods can be applied solely for the study of adherent cells, suitable options to analyze non-adherent cells, such as CTCs, are limited. Moreover, there is a trade-off between throughput and information content [
7]. Certain techniques such as atomic force microscopy (AFM) allow very sensitive measurements but suffer from low-throughput, while microfluidics-based methods like deformability cytometry provide rapid measurements but with limited information content. However, using biophysical properties for practical routine tests to distinguish cells in a sample solution requires both high-throughput and multi-parameter analysis.
Three main features necessary for the targeted practical system are (i) the high-throughput handling of non-adherent cells without compromising (ii) the detection sensitivity for (iii) a multi-parameter analysis. Microfluidics technology is well-suited to handle non-adherent cells and such capabilities have already been demonstrated with high-throughput techniques [
5,
17]. For a practical yet still highly sensitive multi-parameter detection method, on the other hand, microelectromechanical systems (MEMS) technology provides low-noise electrical detection while providing mechanical and electrical stimulation of cells. Integrating MEMS with microfluidics enables the handling and/or analysis of biological samples such as microtubules [
19], DNA [
20], and cells [
21], even for clinical purposes [
22]. MEMS devices with built-in microfluidics simplify biophysical characterization and improve throughput, even though existing examples use external actuation and optical detection [
23,
24]. To achieve the optimal use of MEMS, sensitive harmonic analysis can be combined with practical electrostatic actuation. However, to reach the necessary sensitivity, the MEMS elements have to be operated in air while the cell characterization is performed in liquid.
This study develops a MEMS device with an embedded microfluidic channel to perform multi-parameter biophysical (mechanical and electrical) characterization. Electrostatic actuation allows both single cell stimulation and harmonic analysis for sensitive real-time measurements due to a specific device design, separating the in-liquid biological sample handling from in-air MEMS actions (actuation and detection). Here, we introduce the device features, perform real-time measurements (mechanical and electrical), and demonstrate a proof of concept for single cell handling. This device will allow high-throughput characterization of individual cells in complex biological samples. This could open potential tool alternatives for routine clinical tests for early disease diagnosis with improved cost and time efficiency.
3. Setup and Operation
3.1. Experimental Setup
Experiments are performed on an upright microscope stage (VH-S30B, Keyence Corporation, Osaka, Japan). A long-working distance objective (VH-Z50L, Keyence Corporation) and a camera (Infinity 3, Lumenera Corporation, Ottawa, ON, Canada) are used to monitor the experiments. The fabricated devices are connected to peripheral electronics and fluidic equipment, which are controlled and driven by a LabVIEW (version 16, National Instruments Corporation, Austin, TX, USA) program. Prior to positioning on the microscope stage, the assembled device is mounted on a printed circuit board (PCB) and connected with aluminum wires (
Figure 6a).
Actuating the compressing tip requires applying a potential difference between the combs of the electrostatic actuator. This potential difference is provided with a function generator (33500B, Keysight Technologies Inc., Santa Rosa, CA, USA) and a high voltage amplifier (WMA-100, Falco Systems BV, Katwijk aan Zee, The Netherlands) to maintain higher voltages. As one of the actuation electrodes is also used for electrical sensing, this electrode is grounded virtually by the transimpedance amplifier needed for electrical measurements and the actuation signal is applied on the other electrode (
Figure 6b).
The sensing side has a more complex structure to provide mechanical oscillations for harmonic analysis while simultaneously performing electrical measurements. Phase-lock loops (PLL) are essential to perform harmonic oscillations for mechanical measurements. A lock-in-amplifier (Model 7230, AMETEK, Inc., Berwyn, PA, USA) drives the comb-drive actuations at a constant phase according to the differential capacitive sensor readings. Two stationary electrodes (C1 and C2), forming two identical capacitors with the movable electrode (C0), are connected to the inputs of the lock-in-amplifier after passing through low-noise current-to-voltage (A/V) preamplifiers (Signal Recovery, model 5182). C0, on the other hand, is connected to a power source (provided by the lock-in-amplifier) to be polarized with a constant voltage. The lock-in-amplifier uses sensor measurements to drive the actuators at the resonance frequency. The tip of the sensing area is connected to another lock-in amplifier (HF2LI, Zurich Instruments Ltd., Zurich, Switzerland) for electrical measurements. An electrical signal is applied on the sensing tip, while the compressing tip is connected to a transimpedance preamplifier (Zurich Instruments HF2TA) that feeds the input of the lock-in amplifier.
The channel outlet on the microfluidic PDMS cap is connected to a flow sensor with tubing before reaching the vacuum pump (AF1, Elveflow, Paris, France,
Figure 6a). This equipment is monitored and controlled by the LabVIEW program.
3.2. Liquid Handling
As cell characterization can only be performed in a dedicated area, targeted cells inserted via the channel inlet have to be transported and positioned between the tips. Applying a negative pressure at the outlet of the channel creates a flow and adjusting the pressure level changes the flow speed to control the motion of cells in the channel.
After assembling the PDMS cover and the MEMS device, we connect the outlet of the channel to the pump with tubes (
Figure 7ai). At first, we fill the channel and the connection tube with water (
Figure 7aii–iv). Due to the small dimensions (5 µm gap between tips and the sidewalls), high surface tension at the handling area maintains the stability of the air-liquid interface. As a result, the liquid inside the channel does not leak out (
Figure 7av). Moreover, by injecting another solution in the inlet, we can exchange the solution in the channel within seconds [
20,
30]. This property is important for a variety of purposes, for example washing, surface treatment, and drug testing. This is demonstrated by replacing water inside the channel with a blue-dye solution (
Figure 7b).
3.3. Mechanical Detection Method
By avoiding the usage of the MEMS elements in liquid, we achieve a higher system quality factor when performing sensitive harmonic analyses [
26,
29]. In short, the sensing part of the device oscillates harmonically with a series of comb-drive actuators (0.2 µm of actuation at 3 V
rms). These actuators are driven at the resonance frequency of the system by a lock-in amplifier (Signal Recovery, AMETEK 7270 DSP). Due to the mechanical connection (between the sensor, the actuator and the sensing tip), actuators oscillate the central electrode (C
0) of the differential capacitive sensor. Detecting the capacitance between the movable common electrode (C
0) and the other two fixed electrodes of the differential capacitive sensor (C
1 and C
2), a LabVIEW-controlled PLL keeps the sensing arm at resonance throughput the experiment. Capturing a biological sample between the tips changes the spring constant of the system, which can be detected as a change in the resonance frequency by the PLL driven by the lock-in amplifier.
To improve the detection performance of the displacement sensor, C
0 is polarized with a constant voltage V
0 (3 V) and the fixed electrodes are kept grounded [
28]. During harmonic oscillations, the change in the gap between the mobile and fixed parallel plate electrodes creates dynamic currents that are collected from C
1 and C
2. Amplified with low-noise current-to-voltage preamplifiers (with a gain of 10
8), the sensor outputs are fed into the lock-in-amplifier for real-time measurements.
3.4. Electrical Detection Method
Electrical insulation of the various elements of the sensing area is crucial for simultaneous mechanical and electrical detection. By connecting through the backside silicon, the SiO2 layer keeps the sensing elements mechanically attached while providing electrical access to each individual element. This allows us to apply a sinusoidal signal at the actuator (3 Vrms at the resonance frequency of the system) and to read the sensor outputs in parallel with the electrical measurements performed at the tip of the sensing arm.
The electrical detection is performed between the sensing and the compressing tips. A 1 Vp-p signal is applied on the sensing tip, while the compressing tip is connected to the virtual ground of a transimpedance amplifier. The dynamic current passing through the amplifier is converted to voltage and measured in real-time. Moreover, by sweeping the frequency at the sensing tip, the frequency response of the system can be analyzed.
4. Device Performance and Results
Hereafter, we show some results to investigate the performance of the proposed device. Starting with the frequency response of the device, we performed mechanical and electrical measurements using different sample solutions. Finally, we demonstrated the biomaterial-handling capabilities of the proposed device to confirm the capability of single cell analysis.
4.1. Frequency Response and Real-Time Analysis
To characterize the behavior of the device during harmonic analysis, the frequency response of the system was monitored with the lock-in-amplifier (
Figure 8). Compared to the initial device characteristics (resonance frequency of 1195 Hz with a quality factor (Q-factor) of 8.9), the assembled device (with PDMS cover) showed a slight increase in the resonance frequency (1200 Hz) and a decrease in the Q-factor (6.3) due to the increased damping as a result of the PDMS slab. Filling the channel with liquid also changes the frequency response. Although the effect of the liquid in terms of mass is negligible compared to the total mass of the mobile part of the sensing arm, the surface tension due to the air-liquid interface in the handling region did affect the spring constant of the system. As a result, the resonance frequency was increased to 1220 Hz with a similar Q-factor (6.8).
Observing the changes in the mechanical properties of biological samples requires real-time monitoring of the system response. To achieve this, we performed repeated PLLs controlled by a LabVIEW program. Before comparing different samples and solutions, we tested the stability of the detection method. Although the mechanical characterization of cells requires less than 30 s, we monitored the system for over five min (
Figure S2). Confirmation of the system stability allowed us to perform mechanical measurements in various sample media.
4.2. Mechanical Measurements
Mechanical detection performance of the system was tested by measuring the change in the resonance frequency in solutions with different viscosities and surface properties. We compared the mechanical response of the device in glucose solutions of various concentrations ranging from 0.1% to 10% (w/v). Using the vacuum pump, these solutions were consecutively injected after filling the channel with water. The PLL measurements allowed real-time observation of the changes in the solution between the tips.
Increasing glucose concentration resulted in an increase of the resonance frequency (
Figure 9). Taking the water measurements as the base value, we obtained a resonance frequency shift of 1.4 Hz, 2.4 Hz, and 8.3 Hz for glucose concentrations of 0.1%, 1% and 10%, respectively (
Figure 9a) with stable characteristics (
Figure S3). Similarly, a decrease in amplitude is observed with increasing glucose concentration (
Figure 9b,
Figure S4). Compared to the initial water measurements, a decrease of 5 mV, 7 mV, and 10 mV in the amplitude was observed due to higher damping. These measurements show that the proposed device is capable of observing changes in the mechanical characteristics at the handling area.
4.3. Electrical Measurements
An important property of the proposed device is the ability to perform electrical measurements together with mechanical characterization. To demonstrate this capability, we injected solutions with different ionic strengths in the channel and examined the electrical properties. Similar to the glucose measurements, we filled the channel first with water and then injected different molar concentrations of NaCl (0.1 mM–10 mM) consecutively. Using a lock-in-amplifier, we applied 1 V
p-p to the sensing tip and amplified the signal obtained from the compressing tip with a transimpedance amplifier (with a gain of 10
5). Measurements resulted in increasing voltage waveform amplitudes for increasing ion concentration when the potential difference was applied at 4 kHz (
Figure 10a) indicating an increase in the current passing through the tips. In addition to these real-time measurements (
Figure S5), we could also obtain the frequency response of the system by sweeping the frequency from 100 Hz to 100 kHz (
Figure 10b,
Figure S5).
4.4. Biological Sample Handling
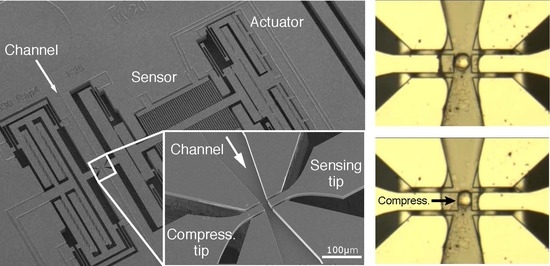
Besides the mechanical and electrical measurements, a key feature of the proposed device is the ability to handle biological samples, e.g., single cells. We examined the single cell handling capability of the device by injecting solutions of fixed cancer cells. The human SUM159PT breast cancer cell line was purchased from Asterand (Detroit, MI, USA). The cells were cultured in F12 medium (Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum (Lonza Group, Basel, Switzerland), streptomycin (100 μg/mL), penicillin (100 units/mL), insulin (5 μg/mL) and hydrocortisone (1 μg/mL) (Invitrogen). Prior to the experiments, subconfluent cell culture were trypsinized, resuspended in single cell solution, and then fixed with 4% Paraformaldehyde (10 min, RT), and rinsed with phosphate buffered saline (PBS).
We controlled the vacuum pump and the compressing actuator to capture single cells. After injecting the cell solution in the channel, we created a flow with pump and applied 110 V at the compressing actuator electrodes to narrow the gap between the tips. This decreases the width of the channel from 20 µm to ~6 µm (
Figure 11a). Thus, cells with a mean diameter of ~16 µm could not pass through. When a cell was stopped at the handling area (
Figure 11b), we immediately stopped the flow and decreased the applied voltage to widen the gap between the tips and position the cell between the tips (
Figure 11c). With the cell thusly captured, we could then apply the compression signal at the compressing actuator (
Figure 11d) to examine the characteristics of the captured cell. After measurements, the compressing tip was moved back to the initial (open) position and the flow was restarted to remove the captured cell and prepare for another cell to characterize.
An important point to note was the stability of the air-liquid interface at the sensing tip. Although the compressing arm moved towards the sensing tip while compressing the cell, the air-liquid interface at the sensing tip was not affected. This stability is a crucial point for reliable and sensitive detection with the proposed device.
5. Conclusions
Separating handling and sensing elements of a microfabricated device is the key to handle and analyze biological samples without compromising precise MEMS performance. We demonstrated a MEMS device with a built-in microfluidic channel to perform single cell biophysical characterization. The built-in channel, requiring no assembly actions between the MEMS and microfluidics elements, not only provides higher-throughput for analysis but also improves sensitivity by allowing integration of microfluidic and MEMS elements at a much finer assembly resolution. Moreover, simultaneous electrical and mechanical measurements allow various parameters to be targeted, such as size, stiffness, viscous losses, membrane fluidity, membrane capacitance, and cytoplasm resistivity. The device is sensitive enough to distinguish differences between liquids according to their mechanical and electrical properties. As demonstrated with cell compression, the device is capable of capturing a single cell and stimulating it mechanically. Sensitive real-time measurements and highly controllable mechanical stimulation allow single cell characterization at different compression levels. Together with the electrical measurements, the device allows for practical multi-parameter analyses of single non-adherent cells to perform routine clinical tests for early disease diagnosis with improved cost and time efficiency.