A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Microfluidic Device and Optimization of Suspension Concentrations
2.2. Optimization of Suspension Concentrations
2.3. Preparation of Cells
2.4. Preparation of Giant Liposomes
2.5. Observation of Electrofusion and Substance Transport between the Cell and the Giant Liposome
3. Results and Discussion
3.1. Relationship between Suspension Concentration and Pair Formation Efficiency
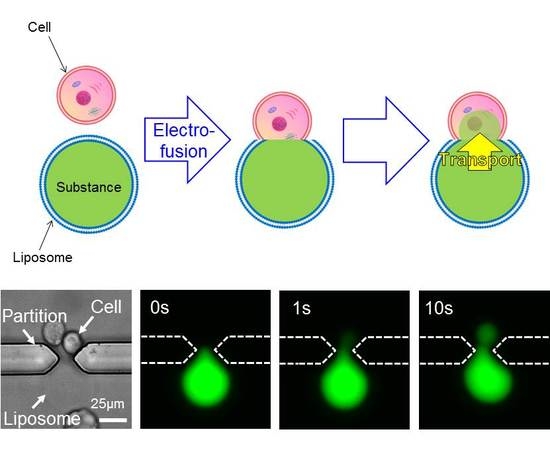
3.2. Electrofusion and Substance Transport between the Cell and the Giant Liposome
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Recillas-Targa, F. Multiple strategies for gene transfer, expression, knockdown, and chromatin influence in mammalian cell lines and transgenic animals. Mol. Biotechnol. 2006, 34, 337–354. [Google Scholar] [CrossRef]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. PNAS 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Kichler, A.; Wallner, G.; Kursa, M.; Ogris, M.; Felzmann, T.; Buchberger, M.; Wagner, E. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997, 4, 409. [Google Scholar] [CrossRef] [PubMed]
- Rémy-Kristensen, A.; Clamme, J.P.; Vuilleumier, C.; Kuhry, J.G.; Mély, Y. Role of endocytosis in the transfection of L929 fibroblasts by polyethylenimine/DNA complexes. Biochim. Biophys. Acta Biomembr. 2001, 1514, 21–32. [Google Scholar]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [PubMed]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Post, J.N.; van Nieuwkasteele, J.W.; ter Braak, P.M.; Kruijer, W.; van den Berg, A. Gene transfer and protein dynamics in stem cells using single cell electroporation in a microfluidic device. Lab Chip 2008, 8, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Natsume, Y.; Toyota, T. Giant Vesicles Containing Microspheres with High Volume Fraction Prepared by Water-in-oil Emulsion Centrifugation. Chem. Lett. 2013, 42, 295–297. [Google Scholar] [CrossRef]
- Nishimura, K.; Suzuki, H.; Toyota, T.; Yomo, T. Size control of giant unilamellar vesicles prepared from inverted emulsion droplets. J. Colloid Interface Sci. 2012, 376, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Oka, M.; Tanaka, T.; Yamazaki, M. A new method for the preparation of giant liposomes in high salt concentrations and growth of protein microcrystals in them. Biochim. Biophys. Acta 2002, 156, 129–134. [Google Scholar] [CrossRef]
- Shirakashi, R.; Sukhorukov, V.L.; Reuss, R.; Schulz, A.; Zimmermann, U. Effects of a Pulse Electric Field on Electrofusion of Giant Unilamellar Vesicle (GUV)-Jurkat Cell. JTST 2012, 7, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.C.; Ogura, T.; Fujiwara, K.; Murata, S.; Nomura, S.M. Introducing Micrometer-Sized Artificial Objects into Live Cells: A Method for Cell–Giant Unilamellar Vesicle Electrofusion. PLoS ONE 2014, 9, e106853. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Washizu, M.; Nanba, T. Novel method of cell fusion in field constriction area in fluid integration circuit. IEEE Trans. Ind. Appl. 1989, 25, 732–737. [Google Scholar] [CrossRef]
- Ito, A. Busshitsu Idou Kaiseki (Analysis of Substance Transport); Asakura Shoten: Tokyo, Japan, 2013; pp. 3–4. ISBN 978-4-25603-1. [Google Scholar]
- Şen, M.; Ino, K.; Ramón-Azcón, J.; Shiku, H.; Matsue, T. Cell pairing using a dielectrophoresis-based device with interdigitated array electrodes. Lab Chip 2013, 13, 3650–3652. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Tomita, M.; Mizutani, F.; Yasukawa, T. Cell Pairing Using Microwell Array Electrodes Based on Dielectrophoresis. Anal. Chem. 2014, 86, 6818–6822. [Google Scholar] [CrossRef] [PubMed]
- Dura, B.; Liu, Y.; Voldman, J. Deformability-based microfluidic cell pairing and fusion. Lab Chip 2014, 14, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Gel, M.; Techaumnat, B.; Oana, H.; Kotera, H.; Washizu, M. Dielectrophoresis-assisted massively parallel cell pairing and fusion based on field constriction created by a micro-orifice array sheet. Electrophoresis 2011, 32, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Miyawaki, A.; Nagai, T. Direct measurement of protein dynamics inside cells using a rationally designed photoconvertible protein. Nat. Methods 2008, 5, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Edward, J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261–270. [Google Scholar] [CrossRef]







| Parameter | Value |
|---|---|
| Diffusion coefficient (µm2/s) | 137 ± 18 |
| Time constant (s) | 1.06 ± 0.10 |
| Fusion ratio (%) | 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyakawa, S.; Uesugi, K.; Morishima, K. A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell. Micromachines 2018, 9, 331. https://doi.org/10.3390/mi9070331
Miyakawa S, Uesugi K, Morishima K. A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell. Micromachines. 2018; 9(7):331. https://doi.org/10.3390/mi9070331
Chicago/Turabian StyleMiyakawa, Shohei, Kaoru Uesugi, and Keisuke Morishima. 2018. "A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell" Micromachines 9, no. 7: 331. https://doi.org/10.3390/mi9070331
APA StyleMiyakawa, S., Uesugi, K., & Morishima, K. (2018). A Closed System for Pico-Liter Order Substance Transport from a Giant Liposome to a Cell. Micromachines, 9(7), 331. https://doi.org/10.3390/mi9070331