Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
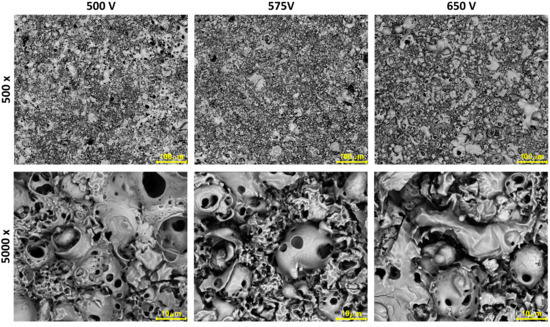
3. Results
4. Discussion
5. Conclusions
- (a)
- The more salt (Mg(NO3)2·6H2O and Zn(NO3)2·6H2O) in electrolyte, the higher the metal-to-phosphorus (Mg/P and Zn/P) ratios that are obtained.
- (b)
- The more salt (Mg(NO3)2·6H2O and/or Zn(NO3)2·6H2O) in electrolyte, the thicker the coating that is formed.
- (c)
- The increase of PEO voltages results in the increase of amorphous phase in the coatings’ structures.
- (d)
- The higher voltage of PEO treatment, the higher are Zn/P and Mg/P ratios in coatings obtained in the electrolytes containing Mg(NO3)2·6H2O and 250 g/L Zn(NO3)2·6H2O.
- (e)
- The top 10 nm layers of the studied coatings are composed of magnesium (Mg2+), zinc (Zn2+), phosphorous (PO43−, or HPO42−, or H2PO4−, or P2O74−), and titanium (Ti4+).
Author Contributions
Funding
Conflicts of Interest
References
- Stojadinović, S.; Jovović, J.; Petković, M.; Vasilić, R.; Konjević, N. Spectroscopic and real-time imaging investigation of tantalum plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 2011, 205, 5406–5413. [Google Scholar] [CrossRef]
- Fidan, S.; Muhaffel, F.; Riool, M.; Cempura, G.; de Boer, L.; Zaat, S.A.J.; Czyrska-Filemonowicz, A.; Cimenoglu, H. Fabrication of oxide layer on zirconium by micro-arc oxidation: Structural and antimicrobial characteristics. Mater. Sci. Eng. C 2017, 71, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Aktug, S.L.; Kutbay, I.; Usta, M. Characterization and formation of bioactive hydroxyapatite coating on commercially pure zirconium by micro arc oxidation. J. Alloys Compd. 2017, 695, 998–1004. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Ćirić, A.; Vasilić, R. Photoluminescence properties of Eu3+ doped HfO2 coatings formed by plasma electrolytic oxidation of hafnium. Opt. Mater. 2018, 77, 19–24. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Vasilić, R. Plasma electrolytic oxidation of hafnium. Int. J. Refract. Met. Hard Mater. 2017, 69, 153–157. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical behavior of plasma electrolytically oxidized niobium in simulated physiological environment. Surf. Coat. Technol. 2018, 344, 121–131. [Google Scholar] [CrossRef]
- Sowa, M.; Worek, J.; Dercz, G.; Korotin, D.M.; Kukharenko, A.I.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Surface characterisation and corrosion behaviour of niobium treated in a Ca- and P-containing solution under sparking conditions. Electrochim. Acta 2016, 198, 91–103. [Google Scholar] [CrossRef]
- Sowa, M.; Woszczak, M.; Kazek-Kęsik, A.; Dercz, G.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Influence of process parameters on plasma electrolytic surface treatment of tantalum for biomedical applications. Appl. Surf. Sci. 2017, 407, 52–63. [Google Scholar] [CrossRef]
- Sowa, M.; Kazek-Kȩsik, A.; Socha, R.P.; Dercz, G.; Michalska, J.; Simka, W. Modification of tantalum surface via plasma electrolytic oxidation in silicate solutions. Electrochim. Acta 2013, 114, 627–636. [Google Scholar] [CrossRef]
- Farhadi, S.S.; Aliofkhazraei, M.; Barati Darband, G.; Abolhasani, A.; Sabour Rouhaghdam, A. Corrosion and wettability of PEO coatings on magnesium by addition of potassium stearate. J. Magnes. Alloys 2017, 5, 210–216. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coat. Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of sealing post-treatments on the corrosion resistance of PEO coated AZ91 magnesium alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Mechanical behaviour of cp-magnesium with duplex hydroxyapatite and PEO coatings. Mater. Sci. Eng. C 2015, 49, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Kasalica, B.; Radić-Perić, J.; Perić, M.; Petković-Benazzouz, M.; Belča, I.; Sarvan, M. The mechanism of evolution of microdischarges at the beginning of the PEO process on aluminum. Surf. Coat. Technol. 2016, 298, 24–32. [Google Scholar] [CrossRef]
- Guan, Y.; Xia, Y.; Li, G. Growth mechanism and corrosion behavior of ceramic coatings on aluminum produced by autocontrol AC pulse PEO. Surf. Coat. Technol. 2008, 202, 4602–4612. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Sandim, H.R.Z. SEM/EDX and XPS studies of niobium after electropolishing. Appl. Surf. Sci. 2012, 263, 357–361. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Rokicki, R.; Prima, F. Nanoindentation and XPS studies of Titanium TNZ alloy after electrochemical polishing in a magnetic field. Materials 2015, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Lahtinen, J.; Hryniewicz, T.; Rzadkiewicz, S. XPS depth profiling analysis of passive surface layers formed on austenitic AISI 304L and AISI 316L SS after high-current-density electropolishing. Surf. Coat. Technol. 2015, 276, 516–520. [Google Scholar] [CrossRef]
- Yao, Z.; Cui, R.; Jiang, Z.; Wang, F. Effects of duty ratio at low frequency on growth mechanism of micro-plasma oxidation ceramic coatings on Ti alloy. Appl. Surf. Sci. 2007, 253, 6778–6783. [Google Scholar] [CrossRef]
- Curran, J.A.; Kalkanci, H.; Magurova, Y.; Clyne, T.W. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surf. Coat. Technol. 2007, 201, 8683–8687. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Chernykh, I.V.; Rudnev, V.S.; Ustinov, A.Y.; Tyrina, L.M.; Nedozorov, P.M.; Dmitrieva, E.E. Catalytically active cobalt-copper-oxide layers on aluminum and titanium. Prot. Metals Phys. Chem. Surf. 2014, 50, 209–217. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.Y.; Nie, X.; Chen, Y. Effects of surface coating preparation and sliding modes on titanium oxide coated titanium alloy for aerospace applications. Int. J. Aerosp. Eng. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- El Achhaba, M.; Schierbaum, K. Structure and hydrogen sensing properties of plasma electrochemically oxidized titanium foils. Procedia Eng. 2012, 47, 566–569. [Google Scholar] [CrossRef]
- Tekin, K.C.; Malayoglu, U.; Shrestha, S. Tribological behaviour of plasma electrolytic oxide coatings on Ti6Al4V and cp-Ti alloys. Surf. Eng. 2016, 32, 435–442. [Google Scholar] [CrossRef]
- Han, Y.; Hong, S.H.; Xu, K.; Cheng, S.; Feng, W.; Li, B.; Wang, Y.; Jia, D.; Zhou, Y.; Göttlicher, M.; et al. Synthesis of nanocrystalline titania films by micro-arc oxidation. Mater. Lett. 2002, 56, 744–747. [Google Scholar] [CrossRef]
- Han, Y.; Xu, K. Photoexcited formation of bone apatite-like coatings on micro-arc oxidized titanium. J. Biomed. Mater. Res. 2004, 71A, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, K.W.; Han, Y.; Luo, Q.; Zhang, D.Q.; Li, X.W.; Zhao, X.; Sun, W.; Zhou, Y.; Göttlicher, M.; et al. Preparation and apatite layer formation of plasma electrolytic oxidation film on titanium for biomedical application. Mater. Lett. 2005, 59, 185–189. [Google Scholar] [CrossRef]
- Song, W.H.; Jun, Y.K.; Han, Y.; Hong, S.H.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Bataillon-Linez, P.; Huang, P.; Zhao, Y.M.; Han, Y.; Traisnel, M.; Xu, K.W.; Hildebrand, H.F. Surface analyses of micro-arc oxidized and hydrothermally treated titanium and effect on osteoblast behavior. J. Biomed. Mater. Res. 2003, 68A, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, Y.W.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.W.; Lee, E.J.; Li, L.H.; Kim, H.E. Hydroxyapatite–TiO2 hybrid coating on Ti implants. J. Biomater. Appl. 2006, 20, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hong, S.H.; Xu, K.; Puz’, A.V.; Gnedenkov, A.S.; Minaev, A.N.; He, J.L.; Jia, D.; Zhou, Y.; Göttlicher, M.; et al. Structure and in vitro bioactivity of titania-based films by micro-arc oxidation. Surf. Coat. Technol. 2003, 168, 249–258. [Google Scholar] [CrossRef]
- Teh, T.H.; Berkani, A.; Mato, S.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K.; Habazaki, H.; Zhou, Y.; Göttlicher, M.; et al. Initial stages of plasma electrolytic oxidation of titanium. Corros. Sci. 2003, 45, 2757–2768. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Vasilyeva, M.S.; Kondrikov, N.B.; Tyrina, L.M.; Feng, J.; Wang, Y.J.; Wu, K.; Habazaki, H.; Zhou, Y.; Göttlicher, M.; et al. Plasma-electrolytic formation, composition and catalytic activity of manganese oxide containing structures on titanium. Appl. Surf. Sci. 2005, 252, 1211–1220. [Google Scholar] [CrossRef]
- Ryu, H.S.; Song, W.H.; Hong, S.H.; Nedozorov, P.M.; Kondrikov, N.B.; Didenko, N.A.; Gerasimenko, A.V.; Habazaki, H.; Zhou, Y.; Göttlicher, M.; et al. Biomimetic apatite induction on Ca-containing titania. Curr. Appl. Phys. 2005, 5, 512–515. [Google Scholar] [CrossRef]
- Chen, J.Z.; Shi, Y.L.; Wang, L.; Yan, F.Y.; Zhang, F.Y.; Janghorban, K.; Wang, Y.; Jia, D.; Zhou, Y.; Göttlicher, M.; et al. Preparation and properties of hydroxyapatite-containing titania coating by micro-arc oxidation. Mater. Lett. 2006, 60, 2538–2543. [Google Scholar] [CrossRef]
- Matykina, E.; Montuori, M.; Gough, J.; Monfort, F.; Berkani, A.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Zhou, Y.; Göttlicher, M.; et al. Spark anodising of titanium for biomedical applications. Trans. IMF 2006, 84, 125–133. [Google Scholar] [CrossRef]
- Han, I.H.; Choi, J.H.; Zhao, B.H.; Baik, H.K.; Lee, I.S.; Minaev, A.N.; He, J.L.; Jia, D.; Zhou, Y.; Göttlicher, M.; et al. Effects of electrical wave form on pore size of micro-arc oxidized TiO2 film. Key Eng. Mater. 2006, 309–311, 375–378. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A.; Avramenko, V.A.; Tsvetnikov, A.K.; Sergienko, V.I.; Kurjavyj, V.G.; Ye, H.; Opra, D.P.; et al. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, X.; Tang, G.; Zhao, N.; Gong, Y.; Zhao, Y.; Zhao, J.; Zhang, X. Corrosion test, cell behavior test, and in vivo study of gradient TiO2 layers produced by compound electrochemical oxidation. J. Biomed. Mater. Res. A 2006, 78, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Habazaki, H.; Onodera, T.; Fushimi, K.; Konno, H.; Toyotake, K.; Zhao, Y.; Zhao, J.; Zhang, X.; Liang, Z.H.; Landers, R.; et al. Spark anodizing of β-Ti alloy for wear-resistant coating. Surf. Coat. Technol. 2007, 201, 8730–8737. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Ryu, J.J.; Sung, Y.M.; Nan, K.; Han, Y.; Ustinov, A.Y.; He, J.L.; Chu, P.K.; Matykina, E.; Landers, R.; et al. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation. Electrochem. Commun. 2007, 9, 1886–1891. [Google Scholar] [CrossRef]
- Ragalevičius, R.; Stalnionis, G.; Niaura, G.; Jagminas, A. Micro-arc oxidation of Ti in a solution of sulfuric acid and Ti+3 salt. Appl. Surf. Sci. 2008, 254, 1608–1613. [Google Scholar] [CrossRef]
- Zhang, W.; Du, K.; Yan, C.; Wang, F.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.J.; Göttlicher, M.; et al. Preparation and characterization of a novel Si-incorporated ceramic film on pure titanium by plasma electrolytic oxidation. Appl. Surf. Sci. 2008, 254, 5216–5223. [Google Scholar] [CrossRef]
- Lebukhova, N.V.; Rudnev, V.S.; Kirichenko, E.A.; Chigrin, P.G.; Lukiyanchuk, I.V.; Yarovaya, T.P.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P.; et al. Effect of the structure of the oxidized titanium surface on the particle size and properties of the deposited copper-molybdate catalyst. Prot. Met. Phys. Chem. Surf. 2016, 52, 1024–1030. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W. Characterisation of calcium- and phosphorus-enriched porous coatings on cp titanium grade 2 fabricated by plasma electrolytic oxidation. Metals 2017, 7, 354. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Pietrzak, K.; Malorny, W.; Salvador Fernandes, J. Characterization of porous phosphate coatings enriched with magnesium or zinc on cp titanium grade 2 under DC plasma electrolytic oxidation. Metals 2018, 8, 112. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Development of plasma electrolytic oxidation for improved Ti6Al4V biomaterial surface properties. Int. J. Adv. Manuf. Technol. 2016, 85, 2425–2437. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P. Investigation of porous coatings obtained on Ti-Nb-Zr-Sn alloy biomaterial by plasma electrolytic oxidation: Characterisation and modelling. Int. J. Adv. Manuf. Technol. 2016, 87, 3497–3512. [Google Scholar] [CrossRef]
- Nelis, T.; Payling, R. Practical guide to glow discharge optical emission spectroscopy. In RSC Analytical Spectroscopy Monographs; Barnett, N.W., Ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Casa Software Ltd. CasaXPS: Processing software for XPS, AES, SIMS and More. 2009. Available online: http://www.casaxps.com (accessed on 25 May 2018).
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Cr/Fe ratio by XPS spectra of magnetoelectropolished AISI 316L SS fitted by Gaussian-Lorentzian shape lines. Teh. Vjesn. 2014, 21, 533–538. [Google Scholar]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database: NIST Standard Reference Database 20, Version 4.1. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 25 May 2018).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Verplancke, R.; Schaubroeck, D.; Van Put, S.; Razi, F.; De Keersmaecker, M.; Adriaens, A.; et al. A multiplexed microfluidic platform for bone marker measurement: A proof-of-concept. Micromachines 2017, 8, 133. [Google Scholar] [CrossRef]
- Erickson, D.; Li, D. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]

















| Electrolytes | Voltages | Ref. |
|---|---|---|
| Na2CO3 and Na3PO4, and (CH3COO)2Ca·H2O | 200–500 V (900 Hz) | [33] |
| (CH3COO)2Ca·H2O and C3H7Na2O6P·5H2O | ||
| Na4P2O7·10H2O and KOH, NaAlO2 | 0–300 V | [34] |
| Na2B4O7·10H2O and (CH3COO)2Mn·4H2O | 450–500 V | [35] |
| (CH3COO)2Ca·H2O | 230 V | [36] |
| (CH3COO)2Ca·H2O and NaH2PO4·2H2O | 260–420 V | [37] |
| NH4H2PO4, CaCl2, NaH2PO4, (CH3COO)2Ca | 0–500 V | [38] |
| KOH | 290 V (100–200 Hz) | [39] |
| KOH | 350 V (1000 Hz) | [40] |
| (NaPO3)6, NaF and NaAlO2 | 150–200 V | [41] |
| K2Al2O4, Na3PO4, NaOH | 400 V | [42] |
| CaCl2 and KH2PO4 | 320–340 V | [43] |
| H2SO4 and Ti2(SO4)3 | 1100 V | [44] |
| Na2(EDTA) and CaO, Ca(H2PO4)2 and Na2SiO3·H2O | 350 V (200 Hz) | [45] |
| Na2SiO3, and NaOH | 280 V | [46] |
| Power Supply | Voltage (V) | Salt | Salt Concentration (g/L) |
|---|---|---|---|
| Three-phase transformer with Graetz Bridge | 450 ± 46 | Mg(NO3)·6H2O | 10 |
| 300 | |||
| 600 | |||
| Zn(NO3)2·6H2O | 10 | ||
| 300 | |||
| 600 | |||
| PWR 1600 H, Multi Range DC Power Supply | 500 | Mg(NO3)2·6H2O | 500 |
| 575 | |||
| 650 | |||
| 500 | Zn(NO3)2·6H2O | 500 | |
| 575 | |||
| 650 | |||
| 500 | Mg(NO3)2·6H2O & Zn(NO3)2·6H2O | 250 + 250 | |
| 575 | |||
| 650 |
| SEM & EDS | XPS | GDOES | XRD |
|---|---|---|---|
| Quanta 250 & 650 FEG (SEM: Field Electron and Iron Company, Hillsboro, OR, USA EDS: Thermo Fisher Scientific, Madison, WI, USA) | SCIENCE SES 2002 (SCIENTA AB, ScientaOmicron, Uppsala, Sweden) | Horiba Scientific GD Profiler 2 (HORIBA Scientific, Palaiseau, France) | Bruker-AXS D8 Advance (BRUKER Corporation, Billerica, MA, USA) |
| High Vacuum | monochromatic (Gammadata-Scienta) Al K(alpha) X-ray source | radio frequency (RF) pulsed source | 2Θ/Θ geometry |
| ESEM mode | (hν = 1486.6 eV) (18.7 mA, 13.02 kV) | pressure: 700 Pa, power: 40 W | radiation CuKα Ni filter |
| EDS Noran System Six | energy step 0.2 eV | frequency: 3000 Hz, duty cycle: 0.25 | voltage 40 kV current 40 mA |
| ETD & BSED detectors | step time 200 ms | anode diameter: 4 mm | step by step mode of 0.014 2Θ with an interval of 0.25 s per step |
| Salt | Concentration | Mean | Stand. Dev. | First Quartile | Third Quartile |
|---|---|---|---|---|---|
| Mg(NO3)2·6H2O | 300 g/L | 0.080 | 0.002 | 0.078 | 0.082 |
| 600 g/L | 0.165 | 0.024 | 0.140 | 0.195 | |
| Zn(NO3)2·6H2O | 300 g/L | 0.054 | 0.004 | 0.050 | 0.058 |
| 600 g/L | 0.089 | 0.016 | 0.075 | 0.105 |
| Ratios | Voltage | Mean | Stand. Dev. | First Quartile | Third Quartile |
|---|---|---|---|---|---|
| Mg/P | 500 V | 0.073 | 0.003 | 0.070 | 0.075 |
| 575 V | 0.084 | 0.004 | 0.081 | 0.088 | |
| 650 V | 0.087 | 0.007 | 0.082 | 0.091 | |
| Zn/P | 500 V | 0.071 | 0.003 | 0.069 | 0.074 |
| 575 V | 0.089 | 0.004 | 0.086 | 0.091 | |
| 650 V | 0.102 | 0.005 | 0.098 | 0.106 | |
| M/P | 500 V | 0.145 | 0.005 | 0.141 | 0.148 |
| 575 V | 0.173 | 0.007 | 0.168 | 0.178 | |
| 650 V | 0.188 | 0.010 | 0.178 | 0.196 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Malorny, W.; Matýsek, D.; Pietrzak, K. Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation. Micromachines 2018, 9, 332. https://doi.org/10.3390/mi9070332
Rokosz K, Hryniewicz T, Gaiaschi S, Chapon P, Raaen S, Malorny W, Matýsek D, Pietrzak K. Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation. Micromachines. 2018; 9(7):332. https://doi.org/10.3390/mi9070332
Chicago/Turabian StyleRokosz, Krzysztof, Tadeusz Hryniewicz, Sofia Gaiaschi, Patrick Chapon, Steinar Raaen, Winfried Malorny, Dalibor Matýsek, and Kornel Pietrzak. 2018. "Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation" Micromachines 9, no. 7: 332. https://doi.org/10.3390/mi9070332
APA StyleRokosz, K., Hryniewicz, T., Gaiaschi, S., Chapon, P., Raaen, S., Malorny, W., Matýsek, D., & Pietrzak, K. (2018). Development of Porous Coatings Enriched with Magnesium and Zinc Obtained by DC Plasma Electrolytic Oxidation. Micromachines, 9(7), 332. https://doi.org/10.3390/mi9070332