Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition
Abstract
:1. Introduction
2. Results
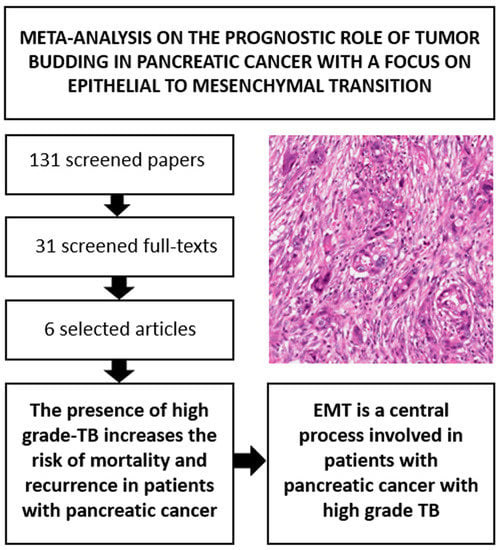
2.1. Search Results
2.2. Study and Patient Characteristics
2.3. Association between High Grade vs. Low Grade Tumor Budding and Survival
2.3.1. All-Cause Mortality (ACM)
2.3.2. Risk of Recurrence (ROR)
2.4. Publication Bias and Meta-Regression Analyses
2.5. Focus on Epithelial to Mesenchymal Transition
3. Discussion
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Data Sources and Literature Search Strategy
4.3. Study Selection
4.4. Data Extraction
4.5. Outcomes
4.6. Assessment of Study Quality
4.7. Data Synthesis and Statistical Analysis
4.8. Focus on Epithelial-to-Mesenchymal Transition with a Systematic Review-Based Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Luchini, C.; Capelli, P.; Scarpa, A. Pancreatic ductal adenocarcinoma and its variants. Surg. Pathol. Clin. 2016, 9, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Saka, B.; Balci, S.; Basturk, O.; Bagci, P.; Postlewait, L.M.; Maithel, S.; Knight, J.; El-Rayes, B.; Kooby, D.; Sarmiento, J.; et al. Pancreatic ductal adenocarcinoma is apread to the peripancreatic soft tissue in the majority of resected cases, rendering the AJCC T-Stage protocol (7th Edition) inapplicable and insignificant: A size-based staging system (pT1: ≤ 2, pT2: > 2 − ≤ 4, pT3: > 4 cm) is more valid and clinically relevant. Ann. Surg. Oncol. 2016, 23, 2010–2018. [Google Scholar] [CrossRef]
- Bal, M.; Rane, S.; Talole, S.; Ramadwar, M.; Deodhar, K.; Patil, P.; Goel, M.; Shrikhande, S. Tumour origin and R1 rates in pancreatic resections: Towards consilience in pathology reporting. Virchows Arch. 2018, 473, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Schorn, S.; Demir, I.E.; Haller, B.; Scheufele, F.; Reyes, C.M.; Tieftrunk, E.; Sargut, M.; Goess, R.; Friess, H.; Ceyhan, G.O. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 105–115. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Pea, A.; Sergi, G.; Manzato, E.; Nottegar, A.; Solmi, M.; Capelli, P.; Scarpa, A. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: A systematic review and meta-analysis of its prognostic significance. Eur. J. Gastroenterol. Hepatol. 2016, 28, 205–209. [Google Scholar] [CrossRef]
- Prall, F. Tumour budding in colorectal carcinoma. Histopathology 2007, 50, 151–162. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [Green Version]
- Rogers, A.C.; Winter, D.C.; Heeney, A.; Gibbons, D.; Lugli, A.; Puppa, G.; Sheahan, K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 2016, 115, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellesso, R.; Luchini, C.; Veronese, N.; Lo Mele, M.; Rosa-Rizzotto, E.; Guido, E.; De Lazzari, F.; Pilati, P.; Farinati, F.; Realdon, S.; et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: A meta-analysis. Hum. Pathol. 2017, 65, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ohike, N.; Coban, I.; Kim, G.E.; Basturk, O.; Tajiri, T.; Krasinskas, A.; Bandyopadhyay, S.; Morohoshi, T.; Shimada, Y.; Kooby, D.A.; et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am. J. Surg. Pathol. 2010, 34, 1417–1424. [Google Scholar] [CrossRef]
- Almangush, A.; Salo, T.; Hagström, J.; Leivo, I. Tumour budding in head and neck squamous cell carcinoma—A systematic review. Histopathology 2014, 65, 587–594. [Google Scholar] [CrossRef]
- Kai, K.; Kohya, N.; Kitahara, K.; Masuda, M.; Miyoshi, A.; Ide, T.; Tokunaga, O.; Miyazaki, K.; Noshiro, H. Tumor budding and dedifferentiation in gallbladder carcinoma: Potential for the prognostic factors in T2 lesions. Virchows Arch. 2011, 459, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.O.; Oh, K.Y.; Shin, W.J.; Yoon, H.J.; Lee, J.I.; Hong, S.D. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum. Pathol. 2018, 80, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Banias, L.; Kovacs, Z.; Jung, I. Epithelial-mesenchymal transition of tumor budding in colorectal cancer: The mystery of CD44-positive stromal cells. Hum. Pathol. 2018, 71, 168–169. [Google Scholar] [CrossRef]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Chouat, E.; Zehani, A.; Chelly, I.; Njima, M.; Maghrebi, H.; Bani, M.A.; Njim, L.; Zakhama, A.; Haouet, S.; Kchir, N. Tumor budding is a prognostic factor linked to epithelial mesenchymal transition in pancreatic ductal adenocarcinoma. Study report and literature review. Pancreatology 2018, 18, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Karamitopoulou, E.; Zlobec, I.; Born, D.; Kondi-Pafiti, A.; Lykoudis, P.; Mellou, A.; Gennatas, K.; Gloor, B.; Lugli, A. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur. J. Cancer 2013, 49, 1032–1039. [Google Scholar] [CrossRef]
- Liu, D.N.; Lv, A.; Tian, Z.H.; Tian, X.Y.; Guan, X.Y.; Dong, B.; Zhao, M.; Hao, C.Y. Superior mesenteric artery margin in pancreaticoduodenectomy for pancreatic adenocarcinoma. Oncotarget 2017, 8, 7766–7776. [Google Scholar] [CrossRef] [PubMed]
- Lohneis, P.; Sinn, M.; Klein, F.; Bischoff, S.; Striefler, J.K.; Wislocka, L.; Sinn, B.V.; Pelzer, U.; Oettle, H.; Riess, H.; et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br. J. Cancer 2018, 118, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Li-Chang, H.H.; Kalloger, S.E.; Peixoto, R.D.; Webber, D.L.; Owen, D.A.; Driman, D.K.; Kirsch, R.; Serra, S.; Scudamore, C.H.; et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 2015, 39, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, L.; Tao, M.; Fu, W.; Xiu, D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin. J. Cancer Res. 2016, 28, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wartenberg, M.; Cibin, S.; Zlobec, I.; Vassella, E.; Eppenberger-Castori, S.; Terracciano, L.; Eichmann, M.D.; Worni, M.; Gloor, B.; Perren, A.; et al. Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance. Clin. Cancer Res. 2018, 24, 4444–4454. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.A.; Zlobec, I.; Wartenberg, M.; Lugli, A.; Gloor, B.; Perren, A.; Karamitopoulou, E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br. J. Cancer 2015, 112, 1944–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, I.; Bronsert, P.; Timme, S.; Werner, M.; Brabletz, T.; Hopt, U.T.; Schilling, O.; Bausch, D.; Keck, T.; Wellner, U.F. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. 1), 78–84. [Google Scholar] [CrossRef] [Green Version]
- Lapshyn, H.; Bolm, L.; Kohler, I.; Werner, M.; Billmann, F.G.; Bausch, D.; Hopt, U.T.; Makowiec, F.; Wittel, U.A.; Keck, T.; et al. Histopathological tumor invasion of the mesenterico-portal vein is characterized by aggressive biology and stroma fibroblast activation. HPB 2017, 19, 67–74. [Google Scholar] [CrossRef]
- Karamitopoulou, E.; Wartenberg, M.; Zlobec, I.; Cibin, S.; Worni, M.; Gloor, B.; Lugli, A. Tumour budding in pancreatic cancer revisited: Validation of the ITBCC scoring system. Histopathology 2018, 73, 137–146. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Role of epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma: Is tumor budding the missing link? Front. Oncol. 2013, 3, 221. [Google Scholar] [CrossRef]
- Luchini, C.; Parcesepe, P.; Mafficini, A.; Nottegar, A.; Parolini, C.; Veronese, N.; Remo, A.; Manfrin, E. Specific expression patterns of epithelial to mesenchymal transition factors in gestational molar disease. Placenta 2015, 36, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, L.; Palmans, S.; Andel, D.; Govaere, O.; Boeckx, B.; Smeets, D.; Galle, E.; Wouters, J.; Barras, D.; Suffiotti, M.; et al. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br. J. Cancer 2017, 116, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Boxberg, M.; Götz, C.; Haidari, S.; Dorfner, C.; Jesinghaus, M.; Drecoll, E.; Boskov, M.; Wolff, K.D.; Weichert, W.; Haller, B.; et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 2018, 73, 559–572. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, L.; Palmans, S.; Sagaert, X. Tumour budding in colorectal cancer: What do we know and what can we do? Virchows Arch. 2016, 468, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Yamada, S.; Koike, M.; Kanda, M.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Nomoto, S.; Fujiwara, M.; Kodera, Y. Epithelial to mesenchymal transition correlates with tumor budding and predicts prognosis in esophageal squamous cell carcinoma. J. Surg. Oncol. 2014, 110, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonradomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 September 2018).
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | N Studies | Risk Ratio (95% CI) | p-Value | Heterogeneity (I2%); Tau2 | Egger Test (p-Value) |
|---|---|---|---|---|---|
| ACM | 5 | 1.46 (1.13–1.88) | 0.004 | 76%; 0.06 | 3.61 (0.08) |
| Parameter | N Cohorts | Hazard Ratio (95% CI) | p-Value | Heterogeneity (I2%); Tau2 | Egger Test (p-Value) |
| ACM | 4 | 2.65 (1.79–3.91) | <0.0001 | 31%; 0.05 | 1.85 (0.29) |
| Parameter | N Cohorts | Risk Ratio (95% CI) | p-Value | Heterogeneity (I2%); Tau2 | Egger Test (p-Value) |
| ROR | 3 | 1.61 (1.05–2.47) | 0.007 | 85%; 0.11 | 4.13 (0.09) |
| References | EMT and Other Important Variables | Main Findings |
|---|---|---|
| Chouat, 2018 [20] | Cytokeratin (AE1/AE3) and vimentin (IHC) | High grade-TB was significantly associated with an increased vimentin expression (p = 0.002). |
| Galván, 2015 [27] | E-cadherin, β-catenin, SNAIL1, ZEB1, ZEB2, N-cadherin, TWIST1 (IHC and mRNA-ISH) | TB cells showed increased levels of ZEB1 (p < 0.0001) and ZEB2 (p = 0.0119) and reduced E-cadherin (p < 0.0001) and β-catenin (p < 0.0001) expression compared with the main tumor. |
| Kohler, 2015 [28] | Cytokeratin (AE1/AE3), E-cadherin, β-catenin, vimentin (IHC) | TB cells showed reduced E-cadherin expression compared with the main tumor. E-cadherin and β-catenin showed reduced expression in the tumor periphery than in the tumor center (p < 0.050). |
| Lapshyn, 2016 [29] | E-cadherin, β-catenin, vimentin (IHC), morphology of cancer-associated fibroblasts | Mesenterico-portal venous tumor infiltration was significantly associated with loss of E-cadherin in tumor buds (p = 0.020), increased vimentin expression (p = 0.03) and activated cancer-associated fibroblasts morphology. |
| Liu, 2017 [22] | Cytokeratin (AE1/AE3) (IHC) | Expression of cytokeratin in tumor budding was significantly lower than in primary tumor (p = 0.001). |
| Wartenberg, 2018 [26] | p63 and “immune-cell” markers | Three PDAC subtypes were identified: the one with the highest grade of TB showed focal p63 expression, frequent CDKN2A and SMAD4 mutations, a reduced presence of T and B cells, was enriched in FOXP3 regulatory T cells, and has the worst outcome. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawlor, R.T.; Veronese, N.; Nottegar, A.; Malleo, G.; Smith, L.; Demurtas, J.; Cheng, L.; Wood, L.D.; Silvestris, N.; Salvia, R.; et al. Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers 2019, 11, 113. https://doi.org/10.3390/cancers11010113
Lawlor RT, Veronese N, Nottegar A, Malleo G, Smith L, Demurtas J, Cheng L, Wood LD, Silvestris N, Salvia R, et al. Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers. 2019; 11(1):113. https://doi.org/10.3390/cancers11010113
Chicago/Turabian StyleLawlor, Rita T., Nicola Veronese, Alessia Nottegar, Giuseppe Malleo, Lee Smith, Jacopo Demurtas, Liang Cheng, Laura D. Wood, Nicola Silvestris, Roberto Salvia, and et al. 2019. "Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition" Cancers 11, no. 1: 113. https://doi.org/10.3390/cancers11010113
APA StyleLawlor, R. T., Veronese, N., Nottegar, A., Malleo, G., Smith, L., Demurtas, J., Cheng, L., Wood, L. D., Silvestris, N., Salvia, R., Scarpa, A., & Luchini, C. (2019). Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers, 11(1), 113. https://doi.org/10.3390/cancers11010113