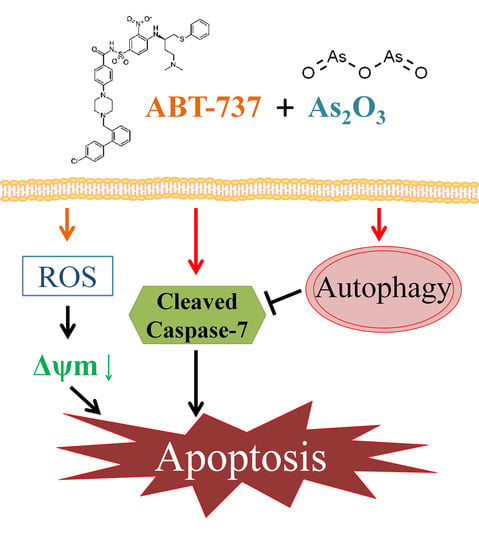
The Application of Arsenic Trioxide in Ameliorating ABT-737 Target Therapy on Uterine Cervical Cancer Cells through Unique Pathways in Cell Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Flow Cytometry for Apoptosis
2.3. Detection of Mitochondrial Membrane Potential (MMP, ΔΨm)
2.4. Detection of Reactive Oxygen Species (ROS)
2.5. Western Blot Analysis
3. Results
3.1. Synergistic Cell Death Induced by Combing ABT-737 with Arsenic Trioxide as Compared to Single Arsenic Trioxide or ABT-737
3.2. Effect of ABT-737 Combined with As2O3 on Annexin V/PI Assay in Cervical Cancer Cells
3.3. Effect of ABT-737 Combined with As2O3 on MMP, ΔΨm
3.4. Effect of ABT-737 Combined with As2O3 on ROS
3.5. Expressions of Anti-Apoptosis Proteins, Cell Cycle Regulated Protein CDK6 and DNA Synthesis TS after ABT-737 and As2O3 Co-Treatment
3.6. Co-Treatment of ABT-737 and As2O3 Induced Anti-Apoptotic Autophagy in SiHa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Willis, S.N.; Adams, J.M. Life in the balance: How BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005, 17, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Yecies, D.; Carlson, N.E.; Deng, J.; Letai, A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010, 115, 3304–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Dai, Y.; Harada, H.; Dent, P.; Grant, S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007, 67, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopleva, M.; Contractor, R.; Tsao, T.; Samudio, I.; Ruvolo, P.P.; Kitada, S.; Deng, X.; Zhai, D.; Shi, Y.X.; Sneed, T.; et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006, 10, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, K.D.; Vandenberg, C.J.; Scott, C.L.; Wei, A.H.; Cory, S.; Huang, D.C.; Roberts, A.W. In Vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17961–17966. [Google Scholar] [CrossRef] [Green Version]
- Kutuk, O.; Letai, A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008, 68, 7985–7994. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Dobrikov, M.; Keir, S.T.; Gromeier, M.; Pastan, I.H.; Reisfeld, R.; Bigner, D.D.; Chandramohan, V. Synergistic antitumor effects of 9.2.27-PE38KDEL and ABT-737 in primary and metastatic brain tumors. PLoS ONE 2019, 14, e0210608. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Zhao, Q. Perifosine and ABT-737 synergistically inhibit lung cancer cells In Vitro and In Vivo. Biochem. Biophys. Res. Commun. 2016, 473, 1170–1176. [Google Scholar] [CrossRef]
- Hwang, E.; Hwang, S.H.; Kim, J.; Park, J.H.; Oh, S.; Kim, Y.A.; Hwang, K.T. ABT-737 ameliorates docetaxel resistance in triple negative breast cancer cell line. Ann. Surg. Treat. Res. 2018, 95, 240–248. [Google Scholar] [CrossRef]
- Antman, K.H. Introduction: The history of arsenic trioxide in cancer therapy. Oncologist 2001, 6 (Suppl. 2), 1–2. [Google Scholar] [CrossRef]
- Kumar, S.; Yedjou, C.G.; Tchounwou, P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Exp. Clin. Cancer Res. 2014, 33, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Qian, H.; Li, Y.; Wang, Y.; Zhang, X.; Liang, X.; Fu, M.; Lin, C. Therapeutic effect of arsenic trioxide (As2O3) on cervical cancer In Vitro and In Vivo through apoptosis induction. Cancer Biol. Ther. 2007, 6, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, L.; Zhan, S.; Chen, L.; Wang, Y.; Zhang, Y.; Du, J.; Wu, Y.; Gu, L. Arsenic Trioxide Suppressed Migration and Angiogenesis by Targeting FOXO3a in Gastric Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Dai, Y.; Zhang, C.; Yang, Y.; Jin, M.; Shan, W.; Shen, J.; Lu, M.; Tang, Z.; Ju, L.; et al. Arsenic trioxide reverses the chemoresistance in hepatocellular carcinoma: A targeted intervention of 14-3-3eta/NF-kappaB feedback loop. J. Exp. Clin. Cancer Res. 2018, 37, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antignani, A.; Youle, R.J. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 2006, 18, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Annis, M.G.; Soucie, E.L.; Dlugosz, P.J.; Cruz-Aguado, J.A.; Penn, L.Z.; Leber, B.; Andrews, D.W. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005, 24, 2096–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlugosz, P.J.; Billen, L.P.; Annis, M.G.; Zhu, W.; Zhang, Z.; Lin, J.; Leber, B.; Andrews, D.W. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006, 25, 2287–2296. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.P.; Wu, W.J.; Ko, J.L.; Wu, T.F.; Yang, S.F.; Wu, C.H.; Yeh, C.M.; Wang, P.H. Effects of ABT-737 combined with irradiation treatment on uterine cervical cancer cells. Oncol. Lett. 2019, 18, 4328–4336. [Google Scholar] [CrossRef]
- Hsin, I.L.; Ou, C.C.; Wu, M.F.; Jan, M.S.; Hsiao, Y.M.; Lin, C.H.; Ko, J.L. GMI, an Immunomodulatory Protein from Ganoderma microsporum, Potentiates Cisplatin-Induced Apoptosis via Autophagy in Lung Cancer Cells. Mol. Pharm. 2015, 12, 1534–1543. [Google Scholar] [CrossRef]
- Hsin, I.L.; Ou, C.C.; Wu, T.C.; Jan, M.S.; Wu, M.F.; Chiu, L.Y.; Lue, K.H.; Ko, J.L. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy 2011, 7, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsin, I.L.; Wang, S.C.; Li, J.R.; Ciou, T.C.; Wu, C.H.; Wu, H.M.; Ko, J.L. Immunomodulatory proteins FIP-gts and chloroquine induce caspase-independent cell death via autophagy for resensitizing cisplatin-resistant urothelial cancer cells. Phytomedicine 2016, 23, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Hsin, I.L.; Hsu, J.C.; Wu, W.J.; Lu, H.J.; Wu, M.F.; Ko, J.L. GMI, a fungal immunomodulatory protein from Ganoderma microsporum, induce apoptosis via beta-catenin suppression in lung cancer cells. Environ. Toxicol. 2018, 33, 955–961. [Google Scholar] [CrossRef]
- St John, J.C.; Amaral, A.; Bowles, E.; Oliveira, J.F.; Lloyd, R.; Freitas, M.; Gray, H.L.; Navara, C.S.; Oliveira, G.; Schatten, G.P.; et al. The analysis of mitochondria and mitochondrial DNA in human embryonic stem cells. Methods Mol. Biol. 2006, 331, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.P.; Pasquale, N.; De, G.; Tan, T.; Ma, J.; Lee, K.B. Core-shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano 2014, 8, 9379–9387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, J.; Baehrecke, E.H. Life, death and autophagy. Nat. Cell Biol. 2018, 20, 1110–1117. [Google Scholar] [CrossRef]
- Filippova, M.; Filippov, V.; Williams, V.M.; Zhang, K.; Kokoza, A.; Bashkirova, S.; Duerksen-Hughes, P. Cellular levels of oxidative stress affect the response of cervical cancer cells to chemotherapeutic agents. Biomed. Res. Int. 2014, 2014, 574659. [Google Scholar] [CrossRef]
- Filippova, M.; Brown-Bryan, T.A.; Casiano, C.A.; Duerksen-Hughes, P.J. The human papillomavirus 16 E6 protein can either protect or further sensitize cells to TNF: Effect of dose. Cell Death Differ. 2005, 12, 1622–1635. [Google Scholar] [CrossRef]
- Liu, S.S.; Chan, K.Y.; Leung, R.C.; Law, H.K.; Leung, T.W.; Ngan, H.Y. Enhancement of the radiosensitivity of cervical cancer cells by overexpressing p73alpha. Mol. Cancer Ther. 2006, 5, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Gottlob, K.; Majewski, N.; Kennedy, S.; Kandel, E.; Robey, R.B.; Hay, N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001, 15, 1406–1418. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Richardson, A.P. The mitochondrial permeability transition: A current perspective on its identity and role in ischaemia/reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 129–141. [Google Scholar] [CrossRef]
- Chevrollier, A.; Loiseau, D.; Reynier, P.; Stepien, G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta 2011, 1807, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Kim, Y.G.; Nam, S.J.; Keam, B.; Kim, T.M.; Jeon, Y.K.; Kim, C.W. Targeting Adenine Nucleotide Translocase-2 (ANT2) to Overcome Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2016, 15, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Vikstrom, I.B.; Slomp, A.; Carrington, E.M.; Moesbergen, L.M.; Chang, C.; Kelly, G.L.; Glaser, S.P.; Jansen, J.H.; Leusen, J.H.; Strasser, A.; et al. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis. 2016, 7, e2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmar, J.; Fesik, S.W. Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol. Ther. 2015, 145, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertz, I.E.; Kusam, S.; Lam, C.; Okamoto, T.; Sandoval, W.; Anderson, D.J.; Helgason, E.; Ernst, J.A.; Eby, M.; Liu, J.; et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011, 471, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Dong, K.; Lin, F.; Wang, X.; Li, B.; Shen, J.J.; Zhang, Q.; Wang, R.; Zhang, H.Z. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother. Pharmacol. 2008, 62, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Ruvolo, V.R.; Wei, J.; Konopleva, M.; Reed, J.C.; Pellecchia, M.; Andreeff, M.; Ruvolo, P.P. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood 2015, 126, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.M.; Min, K.J.; Seo, B.R.; Seo, Y.H.; Jeong, Y.J.; Kwon, T.K. YM155 enhances ABT-737-mediated apoptosis through Mcl-1 downregulation in Mcl-1-overexpressed cancer cells. Mol. Cell Biochem. 2017, 429, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Zhang, X.; He, C.; Qiao, H.; Jiang, X.; Jiang, H.; Sun, X. ABT-737 synergizes with arsenic trioxide to induce apoptosis of gastric carcinoma cells In Vitro and In Vivo. J. Int. Med. Res. 2012, 40, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hilsenbeck, S.; Gazitt, Y. Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood 2003, 101, 4078–4087. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Subbarayan, P.R.; Lee, K.; Ardalan, B. Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant colorectal cancer cell line HT29 In Vitro re-sensitizing cells to 5-FU. Anticancer Res. 2010, 30, 1157–1162. [Google Scholar]
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsin, I.-L.; Chou, Y.-H.; Hung, W.-L.; Ko, J.-L.; Wang, P.-H. The Application of Arsenic Trioxide in Ameliorating ABT-737 Target Therapy on Uterine Cervical Cancer Cells through Unique Pathways in Cell Death. Cancers 2020, 12, 108. https://doi.org/10.3390/cancers12010108
Hsin I-L, Chou Y-H, Hung W-L, Ko J-L, Wang P-H. The Application of Arsenic Trioxide in Ameliorating ABT-737 Target Therapy on Uterine Cervical Cancer Cells through Unique Pathways in Cell Death. Cancers. 2020; 12(1):108. https://doi.org/10.3390/cancers12010108
Chicago/Turabian StyleHsin, I-Lun, Ying-Hsiang Chou, Wei-Li Hung, Jiunn-Liang Ko, and Po-Hui Wang. 2020. "The Application of Arsenic Trioxide in Ameliorating ABT-737 Target Therapy on Uterine Cervical Cancer Cells through Unique Pathways in Cell Death" Cancers 12, no. 1: 108. https://doi.org/10.3390/cancers12010108
APA StyleHsin, I. -L., Chou, Y. -H., Hung, W. -L., Ko, J. -L., & Wang, P. -H. (2020). The Application of Arsenic Trioxide in Ameliorating ABT-737 Target Therapy on Uterine Cervical Cancer Cells through Unique Pathways in Cell Death. Cancers, 12(1), 108. https://doi.org/10.3390/cancers12010108