Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. Cell Densities
2.2. Influence on Survival
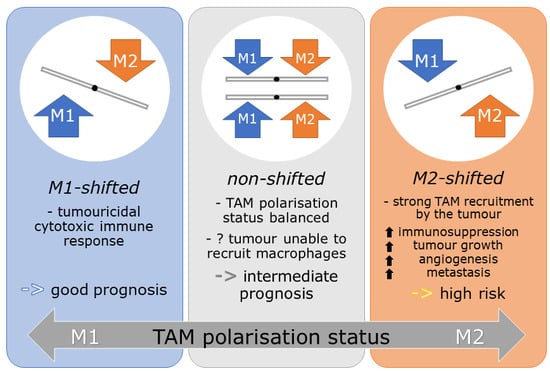
2.3. M-Shift Model
2.4. Influence of M-Shifts on Survival
3. Discussion
4. Materials and Methods
4.1. Breast Cancer Patients and Clinical Data
4.2. TMA Construction and Immunohistochemistry
4.3. Quantification of Macrophages
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer, World Health Organization. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; IARC: Lyon, France, 2018; Volume 263. [Google Scholar]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the united states. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhail, S.; Johnson, S.; Greenberg, D.; Peake, M.; Rous, B. Stage at diagnosis and early mortality from cancer in England. Br. J. Cancer 2015, 112, S108–S115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, S.; Nurkic, S.; Diener-West, M.; Showalter, S.L. Survival after breast-conserving surgery with whole breast or partial breast irradiation in women with early stage breast cancer: A seer data-base analysis. Breast J. 2017, 23, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Abo-Madyan, Y.; Welzel, G.; Sperk, E.; Neumaier, C.; Keller, A.; Clausen, S.; Schneider, F.; Ehmann, M.; Sutterlin, M.; Wenz, F. Single-center long-term results from the randomized phase-3 targit-a trial comparing intraoperative and whole-breast radiation therapy for early breast cancer. Strahlenther. Onkol. 2019, 195, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. Strahlenther. Onkol. 2018, 194, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/m-2 macrophages and the th1/th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D.; Ley, K. M1 and m2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef]
- Tang, R.; Beuvon, F.; Ojeda, M.; Mosseri, V.; Pouillart, P.; Scholl, S. M-csf (monocyte colony stimulating factor) and m-csf receptor expression by breast tumour cells: M-csf mediated recruitment of tumour infiltrating monocytes? J. Cell. Biochem. 1992, 50, 350–356. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, D.; Wang, Q.; Song, B.; Shao, Q.; Sun, J.; Ji, C.; Li, X.; Li, P.; Qu, X. Response gene to complement 32 (rgc-32) expression on m2-polarized and tumor-associated macrophages is m-csf-dependent and enhanced by tumor-derived il-4. Cell. Mol. Immunol. 2015, 12, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized m2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Kong, L.; Zhou, Y.; Bu, H.; Lv, T.; Shi, Y.; Yang, J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J. Exp. Clin. Cancer Res. 2016, 35, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive il-23/il-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. Ccl18 from tumor-associated macrophages promotes breast cancer metastasis via pitpnm3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Ramakrishnan, R.; Altiok, S.; Youn, J.I.; Cheng, P.; Celis, E.; Pisarev, V.; Sherman, S.; Sporn, M.B.; Gabrilovich, D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic t cells in mice. J. Clin. Investig. 2011, 121, 4015–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Liu, L.; Gong, C.Y.; Shi, H.S.; Zeng, Y.H.; Wang, X.Z.; Zhao, Y.W.; Wei, Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Shiao, S.L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res. 2018, 191, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Ponten, F.; Jirstrom, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hamalainen, K.; Tammi, M.; Tammi, R.; Kosma, V.M.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially m2-like (cd163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Honkanen, T.J.; Tikkanen, A.; Karihtala, P.; Makinen, M.; Vayrynen, J.P.; Koivunen, J.P. Prognostic and predictive role of tumour-associated macrophages in her2 positive breast cancer. Sci. Rep. 2019, 9, 10961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Hildebrandt, G.; Potter, R.; Hammer, J.; Lotter, M.; Resch, A.; Sauer, R.; Strnad, V. Accelerated partial breast irradiation with multi-catheter brachytherapy: Local control, side effects and cosmetic outcome for 274 patients. Results of the german-austrian multi-centre trial. Radiother. Oncol. 2007, 82, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.D.; Lewis, C.E.; Whitehouse, R.; Greenall, M.; Clarke, J.; Harris, A.L. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996, 56, 4625–4629. [Google Scholar]
- Gwak, J.M.; Jang, M.H.; Kim, D.I.; Seo, A.N.; Park, S.Y. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS ONE 2015, 10, e0125728. [Google Scholar] [CrossRef]
- Strnad, V.; Hildebrandt, G.; Potter, R.; Hammer, J.; Hindemith, M.; Resch, A.; Spiegl, K.; Lotter, M.; Uter, W.; Bani, M.; et al. Accelerated partial breast irradiation: 5-year results of the german-austrian multicenter phase ii trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 17–24. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of cd68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.; Chu, P.G.; Weiss, L.M. Cd163: A specific marker of macrophages in paraffin-embedded tissue samples. Am. J. Clin. Pathol. 2004, 122, 794–801. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high m1/m2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of m1 and m2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018, 19, 3. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Hang, J.J.; Han, T.; Zhuo, M.; Jiao, F.; Wang, L.W. The m2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumor Biol. 2016, 37, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fushida, S.; Yamamoto, Y.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Miyashita, T.; Tajima, H.; Ninomiya, I.; Munesue, S.; et al. Tumor-associated macrophages of the m2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer 2016, 19, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jin, W.; Chen, C.; Shao, Z.; Wu, J. Tumor associated macrophage x cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS ONE 2012, 7, e41942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvin, S.; Vikhe Patil, E.; Arnesson, L.G.; Oda, H.; Hedayati, E.; Lindstrom, A.; Shabo, I. Differences in intra-tumoral macrophage infiltration and radiotherapy response among intrinsic subtypes in pt1-t2 breast cancers treated with breast-conserving surgery. Virchows Arch. 2019, 475, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Stover, D.G.; Guerriero, J.L.; Dillon, D.; Barry, W.T.; Gjini, E.; Hartl, C.; Lo, W.; Savoie, J.; Brock, J.; et al. The immune microenvironment in hormone receptor-positive breast cancer before and after preoperative chemotherapy. Clin. Cancer Res. 2019, 25, 4644–4655. [Google Scholar] [CrossRef] [Green Version]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. Csf-1r inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Paulus, P.; Stanley, E.R.; Schafer, R.; Abraham, D.; Aharinejad, S. Colony-stimulating factor-1 antibody reverses chemoresistance in human mcf-7 breast cancer xenografts. Cancer Res. 2006, 66, 4349–4356. [Google Scholar] [CrossRef] [Green Version]
- Echarti, A.; Hecht, M.; Buttner-Herold, M.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. Cd8+ and regulatory t cells differentiate tumor immune phenotypes and predict survival in locally advanced head and neck cancer. Cancers 2019, 11, 1398. [Google Scholar] [CrossRef] [Green Version]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, M. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Fasching, P.A.; Gass, P.; Haberle, L.; Volz, B.; Hein, A.; Hack, C.C.; Lux, M.P.; Jud, S.M.; Hartmann, A.; Beckmann, M.W.; et al. Prognostic effect of ki-67 in common clinical subgroups of patients with her2-negative, hormone receptor-positive early breast cancer. Breast Cancer Res. Treat. 2019, 175, 617–625. [Google Scholar] [CrossRef] [PubMed]




| Variables | Groups | |||||
|---|---|---|---|---|---|---|
| Age (years) | Mean: 59.1; | <50: 27 (19.9%); | ≥50: 109 (80.1%) | |||
| T category | pT1mic: 6 (4.4%) | pT1a: 8 (5.9%) | pT1b: 33 (24.3%) | pT1c: 79 (58.1%) | pT2: 10 (7.4%) | |
| N category | N0: 133 (97.8%) | N1: 3 (2.2%) | ||||
| Stage | UICC I: 124 (91.2%) | UICC II: 12 (8.8%) | ||||
| Tumour size (mm) | <10: 34 (25%); | 10–20: 92 (67.6%); | >20: 10 (7.4%) | |||
| Histological grading | G1: 36 (26.5%) | G2: 93 (68.4%) | G3: 4 (2.9%) | n.a. 3 (2.2%) | ||
| Histological typing | Lobular: 20 (14.7%) | No special type: 95 (69.9%) | other: 21 (15.4%) | |||
| Ki67 | <20: 104 (76.5%) | ≥20: 28 (20.6%) | n.a. 4 (2.9%) | |||
| Oestrogen receptor status | Positive: 131 (96.3%) | Negative: 1 (0.7%) | n.a. 4 (2.9%) | |||
| Progesterone receptor status | Positive: 122 (89.7%) | Negative: 11 (8.1%) | n.a. 3 (2.2%) | |||
| Her2 status | Positive: 8 (5.9%) | Negative: 124 (91.2%) | n.a. 4 (2.9%) | |||
| Subtype | Luminal A: 89 (65.4%) | Luminal B: 41 (30.1%) | n.a. 6 (4.4%) | |||
| Hormone therapy | Yes: 122 (89.7%) | No: 14 (10.3%) | ||||
| Chemotherapy | Yes: 9 (6.6%) | No: 127 (93.4%) | ||||
| M1 Groups | Indicators | M2 Cell Densities Central Tumour | M2 Cell Densities Invasive Front | M2 Cell Densities Normal Tissue (Prox) | M2 Cell Densities Normal Tissue (Dist) |
|---|---|---|---|---|---|
| M1 cell densities central tumour | Correlation coefficient | 0.202 * | 0.095 | −0.020 | 0.090 |
| p | 0.028 | 0.322 | 0.872 | 0.412 | |
| n | 119 | 111 | 68 | 86 | |
| M1 cell densities invasive front | Correlation coefficient | 0.121 | 0.100 | 0.133 | 0.110 |
| p | 0.205 | 0.262 | 0.273 | 0.296 | |
| n | 111 | 127 | 70 | 92 | |
| M1 cell densities normal tissue (prox) | Correlation coefficient | −0.097 | 0.100 | 0.409 ** | 0.399 ** |
| p | 0.429 | 0.409 | <0.001 | 0.001 | |
| n | 68 | 70 | 72 | 65 | |
| M1 cell densities normal tissue (dist) | Correlation coefficient | −0.031 | 0.137 | 0.263 * | 0.478 ** |
| p | 0.778 | 0.193 | 0.034 | <0.001 | |
| n | 86 | 92 | 65 | 106 |
| Breast Cancer | Disease-Free Survival | In-Breast Recurrence-Free Survival | ||||
|---|---|---|---|---|---|---|
| Variables | N | 12 yr DFS (%) | p (Log Rank) | N | 12 yr IBRFS (%) | p (Log Rank) |
| Ki67 | ||||||
| <20% | 104 | 86 | 0.057 | 104 | 92 | 0.772 |
| ≥20% | 28 | 77 | 28 | 93 | ||
| Central tumour stromal | ||||||
| M1 low | 53 | 78 | 0.095 | 51 | 88 | 0.241 |
| vs. M1 high | 70 | 87 | 72 | 94 | ||
| M2 low | 23 | 100 | 0.02 | 31 | 100 | 0.033 |
| vs. M2 high | 100 | 79 | 92 | 89 | ||
| M1-shifted + non-shifted | 79 | 89 | 0.01 | 85 | 95 | 0.033 |
| vs. M2-shifted | 44 | 73 | 38 | 84 | ||
| Central tumour intraepithelial | ||||||
| M1 low | 63 | 75 | 0.035 | 61 | 86 | 0.094 |
| vs. M1 high | 56 | 90 | 58 | 96 | ||
| M2 low | 50 | 90 | 0.122 | 54 | 96 | 0.132 |
| vs. M2 high | 69 | 78 | 65 | 88 | ||
| M1-shifted + non-shifted | 85 | 91 | 0.001 | 87 | 96 | 0.004 |
| vs. M2-shifted | 34 | 64 | 32 | 77 | ||
| Invasive front stromal | ||||||
| M1 low | 68 | 77 | 0.011 | 70 | 88 | 0.011 |
| vs. M1 high | 64 | 93 | 62 | 98 | ||
| M2 low | 48 | 95 | 0.035 | 48 | 97 | 0.101 |
| vs. M2 high | 84 | 80 | 84 | 91 | ||
| M1-shifted + non-shifted | 91 | 93 | <0.001 | 90 | 97 | 0.001 |
| vs. M2-shifted | 41 | 68 | 42 | 84 | ||
| Invasive front intraepithelial | ||||||
| M1 low | 69 | 80 | 0.038 | 57 | 86 | 0.025 |
| vs. M1 high | 58 | 90 | 70 | 99 | ||
| M2 low | 72 | 94 | 0.003 | 95 | 96 | 0.004 |
| vs. M2 high | 55 | 74 | 32 | 85 | ||
| M1-shifted + non-shifted | 100 | 90 | <0.001 | 113 | 96 | 0.002 |
| Vs. M2-shifted | 27 | 68 | 15 | 74 | ||
| Breast Cancer | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% C.I. | p | Hazard Ratio | 95% C.I. | p |
| Age (yr) (<50 (n = 26) vs. ≥50 (n = 91)) | 1.757 | 0.352–8.765 | 0.492 | --- | --- | --- |
| pT category (pT1 (n = 108) vs. pT2 (n = 9)) | 1.346 | 0.312–5.809 | 0.691 | --- | --- | --- |
| Stage (UICC I (n = 107) vs. UICC II (n = 10)) | 1.892 | 0.553–6.47 | 0.309 | --- | --- | --- |
| Tumour size (mm) (<20 (n = 102) vs. ≥20 (n = 15)) | 1.109 | 0.044–27.889 | 0.950 | --- | --- | --- |
| Histological grading (G1 (n = 33) vs. G2-3 (n = 84)) | 1.492 | 0.304–7.318 | 0.622 | --- | --- | --- |
| Histological typing (non-lobular (n = 101) vs. lobular (n = 16)) | 0.536 | 0.089–3.22 | 0.495 | --- | --- | --- |
| ER status (negative (n = 1) vs. positive (n = 116)) | * | * | * | --- | --- | --- |
| PR status (negative (n = 11) vs. positive (n = 106)) | 1.152 | 0.065–20.312 | 0.923 | --- | --- | --- |
| Her2 status (negative (n = 110) vs. positive (n = 7)) | 1.357 | 0.137–13.424 | 0.794 | --- | --- | --- |
| Ki67 (<20 (n = 104) vs. ≥20 (n = 28)) | 2.332 | 0.95–5.725 | 0.065 | 1.963 | 0.712–5.41 | 0.193 |
| Hormone therapy (No (n = 12) vs. Yes (n = 105)) | 2.042 | 0.166–25.048 | 0.577 | --- | --- | --- |
| Chemotherapy (No (n = 109) vs. Yes (n = 8)) | 3.831 | 0.374–39.207 | 0.258 | --- | --- | --- |
| TAM shift classification intraepithelial (other (n = 93) vs. M2-shifted (n = 24)) | 4.796 | 1.846–12.461 | 0.001 | 2.890 | 1.058–7.896 | 0.039 |
| TAM shift classification stromal (other (n = 81) vs. M2-shifted (n = 36)) | 9.359 | 2.399–36.509 | 0.001 | 4.223 | 1.408–12.667 | 0.010 |
| Breast Cancer | Stromal TAM Shift Classification (n = 132) | Intraepithelial TAM Shift Classification (n = 127) | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Group | n (total) | M1-Shifted + Non-Shifted | M2-Shifted | p | M1-Shifted + Non-Shifted | M2-Shifted | p |
| Age (yr) | <50 | 27 | 21 (23%) | 6 (15%) | 0.353 | 21 (21%) | 6 (22%) | 1.00 |
| ≥50 | 109 | 70 (77%) | 35 (85%) | 79 (79%) | 21 (78%) | |||
| T Category | pT1 | 126 | 86 (95%) | 36 (88%) | 0.284 | 94 (94%) | 24 (89%) | 0.399 |
| pT2 | 10 | 5 (5%) | 5 (12%) | 6 (6%) | 3 (11%) | |||
| Stage | UICC I | 124 | 86 (95%) | 35 (85%) | 0.095 | 93 (93%) | 24 (89%) | 0.442 |
| UICC II | 12 | 5 (5%) | 6 (15%) | 7 (7%) | 3 (3%) | |||
| Tumour size (mm) | <20 | 120 | 82 (90%) | 34 (83%) | 0.259 | 90 (90%) | 22 (82%) | 0.31 |
| ≥20 | 6 | 9 (10%) | 7 (17%) | 10 (10%) | 5 (18%) | |||
| Histological grading | G1 | 36 | 27 (31%) | 9 (24%) | 0.52 | 30 (31%) | 4 (17%) | 0.208 |
| G2+G3 | 93 | 60 (69%) | 29 (76%) | 66 (69%) | 20 (83%) | |||
| n.a. | 7 | |||||||
| Histological typing | Non lobular | 116 | 78 (86%) | 35 (85%) | 1.00 | 87 (87%) | 23 (85%) | 0.758 |
| Lobular | 20 | 13 (14%) | 6 (15%) | 13 (13%) | 4 (15%) | |||
| DCIS | No | 68 | 44 (54%) | 21 (55%) | 1.00 | 47 (52%) | 16 (67%) | 0.25 |
| Yes | 56 | 38 (46%) | 17 (45%) | 44 (48%) | 8 (33%) | |||
| n.a. | 12 | |||||||
| Ki67 | <20 | 100 | 71 (82%) | 29 (71%) | 0.176 | 78 (81%) | 19 (70%) | 0.285 |
| ≥20 | 28 | 16 (18%) | 12 (29%) | 18 (19%) | 8 (30%) | |||
| n.a. | 4 | |||||||
| ER status | Neg | 1 | 1 (1%) | 0 | 1.00 | 1 (1%) | 0 | 1.00 |
| Pos | 131 | 86 (99%) | 41 (100%) | 95 (99%) | 27 (100%) | |||
| n.a. | 4 | |||||||
| PR status | Neg | 11 | 9 (10%) | 2 (5%) | 0.501 | 7 (7%) | 4 (15%) | 0.253 |
| Pos | 122 | 79 (90%) | 39 (95%) | 90 (93%) | 23 (85%) | |||
| n.a. | 3 | |||||||
| Her2 status | Neg | 124 | 82 (94%) | 38 (93%) | 0.71 | 89 (92%) | 26 (100%) | 0.201 |
| Pos | 8 | 5 (6%) | 3 (7%) | 8 (8%) | 0 | |||
| n.a. | 4 | |||||||
| Subtype | Luminal A | 89 | 59 (68%) | 26 (65%) | 0.688 | 65 (69%) | 17 (63%) | 0.641 |
| Luminal B | 41 | 27 (31%) | 14 (35%) | 29 (31%) | 10 (37%) | |||
| n.a. | 6 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.-C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer. Cancers 2020, 12, 446. https://doi.org/10.3390/cancers12020446
Schnellhardt S, Erber R, Büttner-Herold M, Rosahl M-C, Ott OJ, Strnad V, Beckmann MW, King L, Hartmann A, Fietkau R, et al. Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer. Cancers. 2020; 12(2):446. https://doi.org/10.3390/cancers12020446
Chicago/Turabian StyleSchnellhardt, Sören, Ramona Erber, Maike Büttner-Herold, Marie-Charlotte Rosahl, Oliver J. Ott, Vratislav Strnad, Matthias W. Beckmann, Lillian King, Arndt Hartmann, Rainer Fietkau, and et al. 2020. "Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer" Cancers 12, no. 2: 446. https://doi.org/10.3390/cancers12020446
APA StyleSchnellhardt, S., Erber, R., Büttner-Herold, M., Rosahl, M. -C., Ott, O. J., Strnad, V., Beckmann, M. W., King, L., Hartmann, A., Fietkau, R., & Distel, L. (2020). Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer. Cancers, 12(2), 446. https://doi.org/10.3390/cancers12020446