Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer
Abstract
:1. Introduction
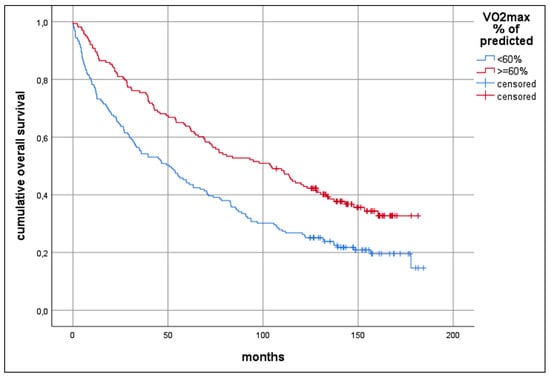
2. Results
3. Discussion
4. Materials and Methods
4.1. Follow-Up and Data Management
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitz, M.W.; Musto, G.; Demers, A.A.; Kliewer, E.V.; Navaratnam, S. Survival and treatment pattern of non-small cell lung cancer over 20 years. J. Thorac. Oncol. 2009, 4, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, M.O.; Fu, P.; Margevicius, S.; Dowlati, A.; Linden, P.A. Five-year survival does not equal cure in non-small cell lung cancer: A Surveillance, Epidemiology, and End Results-based analysis of variables affecting 10- to 18-year survival. J. Thorac. Cardiovasc. Surg. 2012, 143, 1307–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.R.; Henteleff, H.J. Ten-year follow-up of a province-wide cohort of surgical lung cancer patients in Nova Scotia. Can. J. Surg. 2008, 51, 257–262. [Google Scholar] [PubMed]
- Shen-Tu, Y.; Mao, F.; Pan, Y.; Wang, W.; Wang, Z. Lymph node dissection and survival in patients with early stage non-small cell lung cancer: A 10-year cohort study. Medicine 2017, 96, e8356. [Google Scholar] [CrossRef] [PubMed]
- Batevik, R.; Grong, K.; Segadal, L.; Stangeland, L. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: A study of absolute and relative survival over 15 years. Lung Cancer 2005, 47, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Riquet, M.; Mordant, P.; Pricopi, C.; Legras, A.; Foucault, C.; Dujon, A.; Arame, A.; Le Pimpec-Barthes, F. A review of 250 ten-year survivors after pneumonectomy for non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2014, 45, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Bolliger, C.T.; Jordan, P.; Soler, M.; Stulz, P.; Gradel, E.; Skarvan, K. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am. J. Respir. Crit. Care Med. 1995, 151, 1472–1480. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, G.; Lou, N.; Liao, W.; Wang, D. Preoperative Maximal Oxygen Uptake and Exercise-induced Changes in Pulse Oximetry Predict Early Postoperative Respiratory Complications in Lung Cancer Patients. Scand. J. Surg. 2014, 103, 201–208. [Google Scholar] [CrossRef]
- Jensen, M.T.; Holtermann, A.; Bay, H.; Gyntelberg, F. Cardiorespiratory fitness and death from cancer: A 42-year follow-up from the Copenhagen Male Study. Br. J. Sports Med. 2017, 51, 1364–1369. [Google Scholar] [CrossRef]
- Hiraga, T.; Maekura, R.; Okuda, Y.; Okamoto, T.; Hirotani, A.; Kitada, S.; Yoshimura, K.; Yokota, S.; Ito, M.; Ogura, T. Prognostic predictors for survival in patients with COPD using cardiopulmonary exercise testing. Clin. Physiol. Funct. Imaging 2003, 23, 324–331. [Google Scholar] [CrossRef]
- Cote, C.G.; Pinto-Plata, V.; Kasprzyk, K.; Dordelly, L.J.; Celli, B.R. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 2007, 132, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am. Heart J. 2015, 169, 684–692.e1. [Google Scholar] [CrossRef] [PubMed]
- Win, T.; Jackson, A.; Groves, A.M.; Sharples, L.D.; Charman, S.C.; Laroche, C.M. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax 2006, 61, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewen, G.M.; Watson, D.; Kohman, L.; Herndon, J.E., 2nd; Shennib, H.; Kernstine, K.; Olak, J.; Mador, M.J.; Harpole, D.; Sugarbaker, D. Preoperative exercise Vo2 measurement for lung resection candidates: Results of Cancer and Leukemia Group B Protocol 9238. J. Thorac. Oncol. 2007, 2, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licker, M.; Karenovics, W.; Diaper, J.; Frésard, I.; Triponez, F.; Ellenberger, C.; Schorer, R.; Kayser, B.; Bridevaux, P.O. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J. Thorac. Oncol. 2017, 12, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Karenovics, W.; Licker, M.; Ellenberger, C.; Christodoulou, M.; Diaper, J.; Bhatia, C.; Robert, J.; Bridevaux, P.O.; Triponez, F. Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: A randomized controlled study. Eur. J. Cardiothorac. Surg. 2017, 52, 47–54. [Google Scholar] [CrossRef]
- Fresard, I.; Licker, M.; Adler, D.; Lovis, A.; Robert, J.; Karenovics, W.; Diaper, J.; Janssens, J.P.; Triponez, F.; Lador, F.; et al. Preoperative Peak Oxygen Uptake in Lung Cancer Subjects with Neoadjuvant Chemotherapy: A Cross-Sectional Study. Respir. Care 2016, 61, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Pompili, C.; Salati, M.; Refai, M.; Mazzanti, P.; Tiberi, M. Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann. Thorac. Surg. 2014, 98, 238–242. [Google Scholar] [CrossRef]
- Jones, L.W.; Watson, D.; Herndon, J.E., 2nd; Eves, N.D.; Haithcock, B.E.; Loewen, G.; Kohman, L. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 2010, 116, 4825–4832. [Google Scholar] [CrossRef]
- Brutsche, M.H.; Spiliopoulos, A.; Bolliger, C.T.; Licker, M.; Frey, J.G.; Tschopp, J.M. Exercise capacity and extent of resection as predictors of surgical risk in lung cancer. Eur. Respir. J. 2000, 15, 828–832. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.P.; Kinasewitz, G.T.; Tucker, W.Y.; Spillers, W.P.; George, R.B. Exercise capacity as a predictor of post-thoracotomy morbidity. Am. Rev. Respir. Dis. 1984, 129, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Richter Larsen, K.; Svendsen, U.G.; Milman, N.; Brenoe, J.; Petersen, B.N. Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur. Respir. J. 1997, 10, 1559–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzo, R.; Kelley, G.A.; Recchi, L.; Hofman, A.; Sciurba, F. Complications of lung resection and exercise capacity: A meta-analysis. Respir. Med. 2007, 101, 1790–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- British Thoracic Society; Society of Cardiothoarcic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salati, M.; Brunelli, A. Risk Stratification in Lung Resection. Curr. Surg. Rep. 2016, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Cesario, A.; Ferri, L.; Galetta, D. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer 2007, 57, 118–119. [Google Scholar] [CrossRef]
- Milanovic, Z.; Pantelic, S.; Sporis, G.; Mohr, M.; Krustrup, P. Health-Related Physical Fitness in Healthy Untrained Men: Effects on VO2max, Jump Performance and Flexibility of Soccer and Moderate-Intensity Continuous Running. PLoS ONE 2015, 10, e0135319. [Google Scholar] [CrossRef]
- Toth, M.J.; Miller, M.S.; Callahan, D.M.; Sweeny, A.P.; Nunez, I.; Grunberg, S.M.; Der-Torossian, H.; Couch, M.E.; Dittus, K. Molecular mechanisms underlying skeletal muscle weakness in human cancer: Reduced myosin-actin cross-bridge formation and kinetics. J. Appl. Physiol. 2013, 114, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Blair, S.N.; Cheng, Y.; Holder, J.S. Is physical activity or physical fitness more important in defining health benefits? Med. Sci. Sports Exerc. 2001, 33 (Suppl. 6), S379–S399, discussion S419–S420. [Google Scholar] [CrossRef]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.O.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Casaburi, R. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 161–167. [Google Scholar]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar] [CrossRef]
- El-Sherif, A.; Gooding, W.E.; Santos, R.; Pettiford, B.; Ferson, P.F.; Fernando, H.C.; Urda, S.J.; Luketich, J.D.; Landreneau, R.J. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann. Thorac. Surg. 2006, 82, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Sienel, W.; Dango, S.; Kirschbaum, A.; Cucuruz, B.; Hörth, W.; Stremmel, C.; Passlick, B. Sublobar resections in stage IA non-small cell lung cancer: Segmentectomies result in significantly better cancer-related survival than wedge resections. Eur. J. Cardiothorac. Surg. 2008, 33, 728–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; deLeon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar]



| Patients Characteristics I | Mean ± SD | Range |
|---|---|---|
| Age | 63.6 ± 9.4 | 37–86 |
| BMI | 25.9 ± 3.9 | 14.8–38.2 |
| FEV1 mL/min | 2422 ± 607.5 | 960–4650 |
| FEV1 % of predicted | 79.7 ± 16.5 | 36–151 |
| VO2 peak ml/kg/min | 18.3 ± 4.6 | 9.2–51.8 |
| VO2 peak % of predicted | 65.2 ± 18.0 | 32–172 |
| Median observation time overall (months) | 69.2 | 0–184 |
| Median observation time survivors (months) | 149.1 | 125–184 |
| Patients Characteristics II | Number | Percentage (%) |
|---|---|---|
| COPD | 181 | 52.9 |
| Coronary artery disease | 72 | 21.1 |
| Lobectomy, Bi-Lobectomy | 315 | 92.1 |
| Pneumonectomy | 27 | 7.9 |
| Squamous Cell Carcinoma | 112 | 32.7 |
| Adenocarcinoma | 140 | 40.9 |
| Other type of NSCLC (large-cell, polymorphic, spindle-cell) | 90 | 26.3 |
| T0 | 3 | 0.9 |
| T1 | 186 | 54.4 |
| T2 | 128 | 37.4 |
| T3 | 18 | 5.3 |
| T4 | 7 | 2 |
| N0 | 191 | 55.8 |
| N1 | 93 | 27.2 |
| N2 | 58 | 17 |
| Induction Chemotherapy | 43 | 12.6 |
| Adjuvant Chemotherapy | 45 | 13.1 |
| Adjuvant Chemo-Radiotherapy | 5 | 1.4 |
| Adjuvant Radiotherapy | 4 | 1.1 |
| Cause of Death | Number | Percentage (%) |
|---|---|---|
| Lung cancer | 157 | 45.9 |
| Neoplasia other than lung cancer | 22 | 6.4 |
| Other than neoplasia | 66 | 19.3 |
| Chronic cardiac failure | 11 | 3.2 |
| Pneumonia | 9 | 2.6 |
| Myocardial infarction | 8 | 2.3 |
| Renal failure | 7 | 2.0 |
| Stroke | 7 | 2.0 |
| Dementia | 5 | 1.5 |
| Right heart failure | 5 | 1.5 |
| COPD | 3 | 0.9 |
| Decrepitude | 2 | 0.6 |
| Pulmonary embolism | 2 | 0.6 |
| Ileus | 2 | 0.6 |
| Parkinson´s disease | 1 | 0.3 |
| Antibody deficiency syndrome | 1 | 0.3 |
| Multiorgan failure | 1 | 0.3 |
| Peritonitis | 1 | 0.3 |
| Influenza | 1 | 0.3 |
| Total | 245 | 71.6 |
| Parameter | Rho | p-Value |
|---|---|---|
| FEV1 of predicted | 0.2923 | < 0.0001 |
| tumour diameter | −0.0912 | 0.1483 |
| ASA | −0.2532 | < 0.0001 |
| grading | −0.0977 | 0.1212 |
| pT | −0.1709 | 0.0064 |
| pN | −0.1229 | 0.0509 |
| margin | −0.1129 | 0.0731 |
| age | 0.0783 | 0.2143 |
| Criterion | Hazard Ratio | Std. Err. | Z | p > |z| | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| 10 year overall survival | ||||||
| univariable | ||||||
| VO2 peak | 0.959 | 0.016 | −2.48 | 0.013 | 0.928 | 0.991 |
| VO2 peak of predicted | 0.985 | 0.004 | −3.50 | 0.000 | 0.976 | 0.993 |
| multivariable stepwise | ||||||
| pN | 1.487 | 0.159 | 3.71 | 0.000 | 1.205 | 1.835 |
| VO2 peak of predicted | 0.983 | 0.004 | −3.33 | 0.001 | 0.974 | 0.993 |
| Age | 1.029 | 0.009 | 3.32 | 0.001 | 1.012 | 1.047 |
| Neo−adjuvant chemotherapy | 2.250 | 0.529 | 3.45 | 0.001 | 1.418 | 3.569 |
| Tumour diameter | 1.087 | 0.054 | 1.69 | 0.091 | 0.986 | 1.199 |
| 10 year non-tumour-related survival | ||||||
| univariable | ||||||
| VO2 peak | 0.894 | 0.033 | −2.94 | 0.003 | 0.830 | 0.963 |
| VO2 peak of predicted | 0.973 | 0.008 | −2.96 | 0.003 | 0.955 | 0.990 |
| multivariable stepwise | ||||||
| Age | 1.065 | 0.018 | 3.61 | 0.000 | 1.029 | 1.102 |
| VO2 peak of predicted | 0.964 | 0.010 | −3.36 | 0.001 | 0.944 | 0.985 |
| 10 year tumour-related survival | ||||||
| univariable | ||||||
| VO2 peak | 0.975 | 0.017 | −1.37 | 0.171 | 0.940 | 1.010 |
| VO2 peak of predicted | 0.988 | 0.004 | −2.37 | 0.018 | 0.979 | 0.998 |
| multivariable stepwise | ||||||
| pN | 1.558 | 0.191 | 3.61 | 0.000 | 1.224 | 1.984 |
| Neo-adjuvant chemotherapy | 2.875 | 0.710 | 4.28 | 0.000 | 1.772 | 4.666 |
| Tumour diameter | 1.171 | 0.063 | 2.92 | 0.003 | 1.053 | 1.301 |
| Resection margin | 1.976 | 0.856 | 1.57 | 0.116 | 0.844 | 4.622 |
| Pneumonectomy | 0.430 | 0.198 | −1.82 | 0.068 | 0.174 | 1.064 |
| Female gender | 0.725 | 0.136 | −1.71 | 0.087 | 0.501 | 1.048 |
| Impact of Predicted VO2 Peak | Hazard Ratio | Std. Err. | z | p > |z| | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| 10-year overall survival | ||||||
| UICC I | 0.985 | 0.006 | −2.28 | 0.023 | 0.973 | 0.997 |
| UICC II | 0.995 | 0.006 | −0.62 | 0.534 | 0.982 | 1.009 |
| UICC III | 0.966 | 0.010 | −3.09 | 0.002 | 0.945 | 0.987 |
| 10-year non-tumour-related survival | ||||||
| UICC I | 0.982 | 0.011 | −1.60 | 0.110 | 0.960 | 1.004 |
| UICC II | 0.973 | 0.017 | −1.50 | 0.135 | 0.940 | 1.008 |
| UICC III | 0.893 | 0.037 | −2.67 | 0.008 | 0.821 | 0.970 |
| 10-year tumour-related survival | ||||||
| UICC I | 0.986 | 0.007 | −1.82 | 0.068 | 0.971 | 1.001 |
| UICC II | 1.000 | 0.007 | 0.12 | 0.907 | 0.987 | 1.014 |
| UICC III | 0.975 | 0.010 | −2.18 | 0.029 | 0.954 | 0.997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenmann, J.; Fink-Neuboeck, N.; Fediuk, M.; Maier, A.; Kovacs, G.; Balic, M.; Smolle, J.; Smolle-Juettner, F.M. Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer. Cancers 2020, 12, 836. https://doi.org/10.3390/cancers12040836
Lindenmann J, Fink-Neuboeck N, Fediuk M, Maier A, Kovacs G, Balic M, Smolle J, Smolle-Juettner FM. Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer. Cancers. 2020; 12(4):836. https://doi.org/10.3390/cancers12040836
Chicago/Turabian StyleLindenmann, Joerg, Nicole Fink-Neuboeck, Melanie Fediuk, Alfred Maier, Gabor Kovacs, Marija Balic, Josef Smolle, and Freyja Maria Smolle-Juettner. 2020. "Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer" Cancers 12, no. 4: 836. https://doi.org/10.3390/cancers12040836
APA StyleLindenmann, J., Fink-Neuboeck, N., Fediuk, M., Maier, A., Kovacs, G., Balic, M., Smolle, J., & Smolle-Juettner, F. M. (2020). Preoperative Peak Oxygen Consumption: A Predictor of Survival in Resected Lung Cancer. Cancers, 12(4), 836. https://doi.org/10.3390/cancers12040836