Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy
Abstract
:1. Introduction
2. Results
2.1. Cell Death Induction by Radiotherapy in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines
2.2. Cell Death Induction by Hyperthermia in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines
No Significant Cell Death Induction by Conventional Warm-Water Heating but by Microwave Heating
2.3. Cell Death Induction by Hyperthermia and Radiotherapy in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines
Conventional Warm-Water Hyperthermia Barely Induces Further Cell Death in Combination with Normo- or Hypofractionated Irradiation, but Microwave Heating Has Additive Cell Killing Effects
2.4. Release of Danger Signal HSP70 in the Supernatant Following Radiotherapy and/or Hyperthermia
2.4.1. The Danger Signal HSP70 is Significantly Increased after Radiotherapy
2.4.2. Significantly Increased Release of Danger Signal HSP70 Directly after Hyperthermia in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines
2.4.3. Release of Danger Signal HSP70 after Hyperthermia and Radiotherapy on Day 3 and Day 5
2.5. Impact of Radiotherapy and Hyperthermia on the Expression of Immune Checkpoint Molecules
2.5.1. Modulation of Immune Checkpoint Molecules on the Tumor Cell Surface after Radiotherapy
2.5.2. Expression of Immune Checkpoint Molecules on the Tumor Cell Surface after Hyperthermia
2.5.3. Modulation of Immune Suppressive Checkpoint Molecules on the Tumor Cell Surface after Hyperthermia and Radiotherapy
2.5.4. Modulation of Immune Stimulatory Checkpoint Molecules on the Tumor Cell Surface after Hyperthermia and Radiotherapy
2.6. Numerical Simulations to Demonstrate Comparable Heating Conditions of CH and MH
3. Discussion
3.1. Augmenting Breast Cancer Cell Death by Microwave-Based Hyperthermia and Radiotherapy
3.2. Different Temperature Ranges of Hyperthermia Might be Most Beneficial for Immunomodulation
3.3. Adding Hyperthermia to Radiotherapy Dynamically and Individually Affects the Expression of Immune Checkpoint Molecules on Breast Cancer Cells
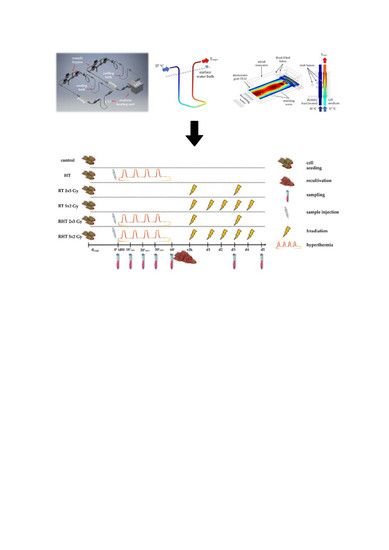
4. Materials and Methods
4.1. Closed-Loop System for Heat Treatments of Tumor Cells
4.2. Numerical Simulations and Modeling of the Heating System
4.3. Cell Lines and Cultivation
4.4. Treatments and Sampling
4.5. Cell Death Detection by AnnexinV/PI Staining
4.6. Detection of Heat Shock Protein 70 (HSP70) by ELISA
4.7. Detection of Immune Checkpoint Molecule and EGFR Expression by Multicolor Flow Cytometry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Gating and Calculation Strategy for Analyses of Expression of Immune Checkpoint Molecules on The Tumor Cell Surface following Treatment with HT and/or RT






References
- Vesely, M.D.; Schreiber, R.D. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Rückert, M.; Deloch, L.; Fietkau, R.; Frey, B.; Hecht, M.; Gaipl, U.S. Immunmodulierende Eigenschaften von Radiotherapie als Basis für wohldurchdachte Radioimmuntherapien. Strahlenther. Onkol. 2018, 194, 509–519. [Google Scholar] [CrossRef]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Z.; Yu, Q.; Wang, X.; Bian, M.; Yu, Z.; Yu, J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019, 9, 14356. [Google Scholar] [CrossRef] [Green Version]
- Timaner, M.; Kotsofruk, R.; Raviv, Z.; Magidey, K.; Shechter, D.; Kan, T.; Nevelsky, A.; Daniel, S.; de Vries, E.G.E.; Zhang, T.; et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene 2020, 39, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Kötter, B.; Frey, B.; Winderl, M.; Rubner, Y.; Scheithauer, H.; Sieber, R.; Fietkau, R.; Gaipl, U.S. The in vitro immunogenic potential of caspase-3 proficient breast cancer cells with basal low immunogenicity is increased by hypofractionated irradiation. Radiat. Oncol. 2015, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Hurwitz, M.; Stauffer, P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin. Oncol. 2014, 41, 714–729. [Google Scholar] [CrossRef] [Green Version]
- Paulides, M.M.; Dobsicek Trefna, H.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency- andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Notter, M.; Thomsen, A.R.; Nitsche, M.; Hermann, R.M.; Wolff, H.A.; Habl, G.; Münch, K.; Grosu, A.-L.; Vaupel, P. Combined wIRA-Hyperthermia and Hypofractionated Re-Irradiation in the Treatment of Locally Recurrent Breast Cancer: Evaluation of Therapeutic Outcome Based on a Novel Size Classification. Cancers 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, W.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Krug, D.; Piroth, M.D.; Sautter-Bihl, M.-L.; Sedlmayer, F.; et al. DEGRO practical guidelines for radiotherapy of breast cancer VI: Therapy of locoregional breast cancer recurrences. Strahlenther. Onkol. 2016, 192, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Willerding, L.; Limmer, S.; Hossann, M.; Zengerle, A.; Wachholz, K.; ten Hagen, T.L.M.; Koning, G.A.; Sroka, R.; Lindner, L.H.; Peller, M. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J. Control. Release 2016, 222, 47–55. [Google Scholar] [CrossRef]
- Trefná, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; van Holthe, N.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [Green Version]
- Maluta, S.; Kolff, M.W. Role of Hyperthermia in Breast Cancer Locoregional Recurrence: A Review. Breast Care 2015, 10, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Schildkopf, P.; Holmer, R.; Sieber, R.; Ott, O.J.; Janko, C.; Mantel, F.; Frey, B.; Fietkau, R.; Gaipl, U.S. Hyperthermia in combination with X-irradiation induces inflammatory forms of cell death. Autoimmunity 2009, 42, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Deloch, L.; Derer, A.; Hartmann, J.; Frey, B.; Fietkau, R.; Gaipl, U.S. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front. Oncol. 2016, 6, 141. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, C.M.; Crezee, J.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A.; Bel, A.; Kok, H.P. The effect of time interval between radiotherapy and hyperthermia on planned equivalent radiation dose. Int. J. Hyperth. 2018, 34, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; ten Cate, R.; van Leeuwen, C.M.; Rodermond, H.M.; de Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P.; et al. Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Colle, C.; Weidner, N.; Heinrich, V.; Brucker, S.; Hahn, M.; MacMillan, K.; Lamprecht, U.; Gaupp, S.; Voigt, O.; Zips, D. Hyperthermie und Rebestrahlung der Brustwand bei rezidivierendem Brustkrebs: Eine prospektive Beobachtungsstudie. Strahlenther. Onkol. 2019, 195, 318–326. [Google Scholar] [CrossRef]
- Brunt, A.M.; Haviland, J.; Sydenham, M.; Algurafi, H.; Alhasso, A.; Bliss, P.; Bloomfield, D.; Emson, M.; Goodman, A.; Harnett, A.; et al. FAST Phase III RCT of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: 10-Year Results (CRUKE/04/015). Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1603–1604. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Huang, F.; Chen, X.; Cao, X.; Yu, J. The long-term outcome of adjuvant hypofractionated radiotherapy and conventional fractionated radiotherapy after breast-conserving surgery for early breast cancer: A prospective analysis of 107 cases. J. Thorac. Dis. 2017, 9, 3840–3850. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; Zhao, M.; Rodríguez, N.; Granado, R.; Foro, P.; Reig, A.; Membrive, I.; Algara, M. Once-Weekly Hypofractionated Radiotherapy for Breast Cancer in Elderly Patients: Efficacy and Tolerance in 486 Patients. BioMed Res. Int. 2018, 2018, 8321871. [Google Scholar] [CrossRef]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Kok, H.P.; Crezee, J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int. J. Hyperth. 2017, 33, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werthmöller, N.; Frey, B.; Rückert, M.; Lotter, M.; Fietkau, R.; Gaipl, U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int. J. Hyperth. 2016, 32, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, J.A.; Romero-Sierra, C.; Davie, S.J. Non-thermal Effects of Microwave Radiation on Birds. Nature 1967, 216, 1139. [Google Scholar] [CrossRef]
- De La Hoz, A.; Díaz-Ortiz, A.; Moreno, A. Review on Non-Thermal Effects of Microwave Irradiation in Organic Synthesis. J. Microw. Power Electromagn. Energy 2006, 41, 45–66. [Google Scholar] [CrossRef]
- Barnes, F.S.; Greenebaum, B. Biological and Medical Aspects of Electromagnetic Fields, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 0-8493-9538-0. [Google Scholar]
- Zhao, Y.-Y.; Wu, Q.; Wu, Z.-B.; Zhang, J.-J.; Zhu, L.-C.; Yang, Y.; Ma, S.-L.; Zhang, S.-R. Microwave hyperthermia promotes caspase-3-dependent apoptosis and induces G2/M checkpoint arrest via the ATM pathway in non-small cell lung cancer cells. Int. J. Oncol. 2018, 53, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Rosin, A.; Hader, M.; Drescher, C.; Suntinger, M.; Gerdes, T.; Willert-Porada, M.; Gaipl, U.S.; Frey, B. Comparative study and simulation of tumor cell inactivation by microwave and conventional heating. COMPEL 2018, 37, 1893–1904. [Google Scholar] [CrossRef]
- Kouloulias, V.; Triantopoulou, S.; Uzunoglou, N.; Pistevou-Gompaki, K.; Barich, A.; Zygogianni, A.; Kyrgias, G.; Kardamakis, D.; Pectasidis, D.; Kouvaris, J. Hyperthermia Is Now Included in the NCCN Clinical Practice Guidelines for Breast Cancer Recurrences: An Analysis of Existing Data. Breast Care 2015, 10, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Kotchapradit, S.; Thongsopa, C.; Thosdeekoraphat, T. Analysis and Design of Microwave Dielectric Heating with Curved Plate Applicator for Deep Hyperthermia in Breast Cancer Treatment. Radioengineering 2019, 28, 703–713. [Google Scholar] [CrossRef]
- Elkayal, H.A.; Ismail, N.E.; Lotfy, M. Microwaves for breast cancer treatments. Alex. Eng. J. 2015, 54, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Derer, A.; Deloch, L.; Rubner, Y.; Fietkau, R.; Frey, B.; Gaipl, U.S. Radio-Immunotherapy-Induced Immunogenic Cancer Cells as Basis for Induction of Systemic Anti-Tumor Immune Responses—Pre-Clinical Evidence and Ongoing Clinical Applications. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witulla, B.; Goerig, N.; Putz, F.; Frey, B.; Engelhorn, T.; Dörfler, A.; Uder, M.; Fietkau, R.; Bert, C.; Laun, F.B. On PTV definition for glioblastoma based on fiber tracking of diffusion tensor imaging data. PLoS ONE 2020, 15, e0227146. [Google Scholar] [CrossRef] [PubMed]
- van Rhoon, G.C. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef]
- Rau, B.; Wust, P.; Tilly, W.; Gellermann, J.; Harder, C.; Riess, H.; Budach, V.; Felix, R.; Schlag, P.M. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 381–391. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.M.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; van der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- Bicher, H.I.; Wolfstein, R.S.; Lewinsky, B.S.; Frey, H.S.; Fingerhut, A.G. Microwave hyperthermia as an adjunct to radiation therapy: Summary experience of 256 multifraction treatment cases. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1667–1671. [Google Scholar] [CrossRef]
- Crezee, H.; Kok, H.P.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A. The Impact of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. The Effect of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in 400 Locally Advanced Cervical Carcinoma Patients. Front. Oncol. 2019, 9, 134. [Google Scholar] [CrossRef]
- Rühle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry. Int. J. Mol. Sci. 2016, 17, 1316. [Google Scholar] [CrossRef] [Green Version]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther. Onkol. 2009, 185, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-L.; Huang, C.-C.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Hsia, C.-C.; Andocs, G.; Wang, H.-E.; Chi, K.-H. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget 2016, 7, 84082–84092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, Y.-W.; Huang, C.-C.; Yang, K.-L.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Andocs, G.; Szasz, A.; Li, W.-T.; Chi, K.-H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, D.F.; Bilek, M.M.; McKenzie, D.R. Non-thermal effects in the microwave induced unfolding of proteins observed by chaperone binding. Bioelectromagnetics 2008, 29, 324–330. [Google Scholar] [CrossRef]
- Frey, B.; Rückert, M.; Weber, J.; Mayr, X.; Derer, A.; Lotter, M.; Bert, C.; Rödel, F.; Fietkau, R.; Gaipl, U.S. Hypofractionated Irradiation Has Immune Stimulatory Potential and Induces a Timely Restricted Infiltration of Immune Cells in Colon Cancer Tumors. Front. Immunol. 2017, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy-immunotherapy combinations: Perspectives and challenges. Mol. Oncol. 2020. [Google Scholar] [CrossRef]
- Derer, A.; Spiljar, M.; Bäumler, M.; Hecht, M.; Fietkau, R.; Frey, B.; Gaipl, U.S. Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.-R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8+ Tumor-Infiltrating Lymphocytes Identified Patients with Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer 2015, 112, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, J.M.; Erdag, G.; Smolkin, M.E.; Deacon, D.H.; Patterson, J.W.; Chen, L.; Bullock, T.N.; Slingluff, C.L. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology 2016, 5, e1235107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Vilain, R.E.; Menzies, A.M.; Wilmott, J.S.; Kakavand, H.; Madore, J.; Guminski, A.; Liniker, E.; Kong, B.Y.; Cooper, A.J.; Howle, J.R.; et al. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin. Cancer Res. 2017, 23, 5024–5033. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.; Bai, I.; Nguyen, K.; Bredno, J.; Vennapusa, B.; Fonstad, R.; Agarwal, S.; Patil, S.; Little, E.; Koeppen, H.; et al. P2.01-043 Pathologist Agreement Rates of PD-L1 Tumor and Immune Cell Quantitation Using Digital Read, Field-Of-View, and Whole Tumor Image Analysis. J. Thorac. Oncol. 2017, 12, S811–S812. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Ren, Y.; Wang, Z. Programmed death 1 Ligand 1 expression in breast cancer and its association with patients’ clinical parameters. J. Cancer Res. Ther. 2018, 14, 150–154. [Google Scholar] [CrossRef]
- Chu, D.-T.; Bac, N.D.; Nguyen, K.-H.; Le Tien, N.B.; van Thanh, V.; Nga, V.T.; Ngoc, V.T.N.; Anh Dao, D.T.; Le Hoan, N.; Hung, N.P.; et al. An Update on Anti-CD137 Antibodies in Immunotherapies for Cancer. Int. J. Mol. Sci. 2019, 20, 1822. [Google Scholar] [CrossRef] [Green Version]
- Malamas, A.S.; Hammond, S.A.; Schlom, J.; Hodge, J.W. Combination therapy with an OX40L fusion protein and a vaccine targeting the transcription factor twist inhibits metastasis in a murine model of breast cancer. Oncotarget 2017, 8, 90825–90841. [Google Scholar] [CrossRef] [Green Version]
- Maennling, A.E.; Tur, M.K.; Niebert, M.; Klockenbring, T.; Zeppernick, F.; Gattenlöhner, S.; Meinhold-Heerlein, I.; Hussain, A.F. Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [Green Version]
- Seitz, C.; Rückert, M.; Deloch, L.; Weiss, E.-M.; Utz, S.; Izydor, M.; Ebel, N.; Schlücker, E.; Fietkau, R.; Gaipl, U.S.; et al. Tumor Cell-Based Vaccine Generated with High Hydrostatic Pressure Synergizes with Radiotherapy by Generating a Favorable Anti-Tumor Immune Microenvironment. Front. Oncol. 2019, 9, 805. [Google Scholar] [CrossRef]
- von Hippel, A.R. Dielectric Materials and Applications, 2nd ed.; Artech House: Boston, MA, USA, 1995; ISBN 1580531237. [Google Scholar]
- Jänicke, R.U.; Ng, P.; Sprengart, M.L.; Porter, A.G. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 1998, 273, 15540–15545. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.C.; Locke, E.R.; Soule, H.D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J. Biol. Chem. 1973, 248, 6251–6253. [Google Scholar]
- Lacroix, M.; Leclercq, G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res. Treat. 2004, 83, 249–289. [Google Scholar] [CrossRef]
- Young, R.K.; Cailleau, R.M.; Mackay, B.; Reeves, W.J. Establishment of epithelial cell line MDA-MB-157 from metastatic pleural effusion of human breast carcinoma. In Vitro 1974, 9, 239–245. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Gaipl, U.S.; Kuenkele, S.; Voll, R.E.; Beyer, T.D.; Kolowos, W.; Heyder, P.; Kalden, J.R.; Herrmann, M. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001, 8, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Hollville, E.; Martin, S.J. Measuring apoptosis by microscopy and flow cytometry. Methods 2013, 61, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Dobšíček Trefná, H.; Crezee, J.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Nadobny, J.; Hartmann, J.; Lomax, N.; Abdel-Rahman, S.; et al. Leitlinien zur Qualitätssicherung der lokalen Hyperthermie in klinischen Studien: II. Technische Anforderungen an Heizgeräte. Strahlenther. Onkol. 2017, 193, 351–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]


















| Marker | Laser Color | Manufacturer | Cat. Nr. | µL Per Well |
|---|---|---|---|---|
| PD-L1 (CD 274) | BV 605 | BioLegend | 329724 | 0.5 1 |
| PD-L2 (CD 273) | APC | BioLegend | 345508 | 0.5 1 |
| EGF-Receptor | PE | BioLegend | 352904 | 0.5 1 |
| ICOS-L (CD 275) | BV 421 | BD Bioscience | 564276 | 0.5 1 |
| HVEM (CD 270) | APC | BioLegend | 318808 | 0.5 2 |
| OX40-L (CD252) | PE | BioLegend | 326308 | 0.5 2 |
| TNFRSF9 (CD137-L) | BV 421 | BioLegend | 311508 | 0.5 2 |
| CD27-L (CD70) | FITC | BioLegend | 355106 | 0.5 2 |
| Zombie NIR | APC-A750 | BioLegend | 423105 | 0.1 1,2,3 |
| FACS-buffer | 2% FBS in DPBS (sterile) | 97.9 1,2/99.9 3 | ||
| Parameter | 25 °C | 35 °C | 45 °C | 55 °C |
|---|---|---|---|---|
| 76.7 | 74.0 | 70.7 | 67.5 | |
| 12.04 | 9.398 | 7.494 | 6.008 |
| Parameter | Stainless Steel (CH) | Stainless Steel (MH) | Quartz Glass (MH) | Air (MH & CH) |
|---|---|---|---|---|
| Relative permeability, µr (-) | 1 | 1 | 1 | 1 |
| Electric conductivity, σ (S/m) | — | 4 · 106 | — | 0.85 |
| Relative permittivity, εr (-) | — | — | 3.78 - j2 · 10−4 | 1 |
| Density, (kg/m³) | 7800 | 7850 | 2200 | 1.2 |
| Heat conductivity, λ (W/mK) | 15 | 44.5 | 1.1 | 0.026 |
| Heat capacity, cp (J/kgK) | 420 | 475 | 480 | 1006 |
| Parameter | Levels | Unit |
|---|---|---|
| Ttarget | 37.0, 39.0, 41.0, 44.0 | °C |
| m | 2.0 | mL/s |
| teff * | 10 1, 20 1, 30 1, 60 | min |
| normo(fractionation) 2 | 5 fractions of 2 Gy consecutively | |
| hypo(fractionation) 2 | 2 fractions of 5 Gy on d0 and d3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hader, M.; Savcigil, D.P.; Rosin, A.; Ponfick, P.; Gekle, S.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers 2020, 12, 1082. https://doi.org/10.3390/cancers12051082
Hader M, Savcigil DP, Rosin A, Ponfick P, Gekle S, Wadepohl M, Bekeschus S, Fietkau R, Frey B, Schlücker E, et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers. 2020; 12(5):1082. https://doi.org/10.3390/cancers12051082
Chicago/Turabian StyleHader, Michael, Deniz Pinar Savcigil, Andreas Rosin, Philipp Ponfick, Stephan Gekle, Martin Wadepohl, Sander Bekeschus, Rainer Fietkau, Benjamin Frey, Eberhard Schlücker, and et al. 2020. "Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy" Cancers 12, no. 5: 1082. https://doi.org/10.3390/cancers12051082
APA StyleHader, M., Savcigil, D. P., Rosin, A., Ponfick, P., Gekle, S., Wadepohl, M., Bekeschus, S., Fietkau, R., Frey, B., Schlücker, E., & Gaipl, U. S. (2020). Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers, 12(5), 1082. https://doi.org/10.3390/cancers12051082