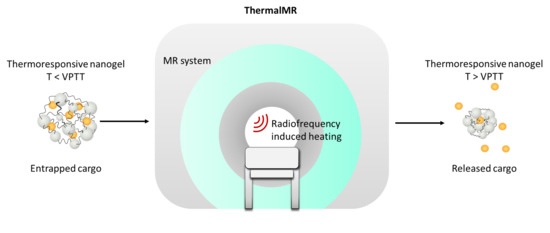
Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study
Abstract
:1. Introduction
2. Results
2.1. Temperature Simulations of the Phantom
2.2. RF Heating of the Experimental Phantom
2.3. Thermoresponsive Nanogel Synthesis and Characterization
2.4. Nanogel Release Profile using a Water Bath for Temperature Modulation
2.5. Nanogel Release Profile using ThermalMR for Temperature Modulation
3. Discussion
4. Materials and Methods
4.1. Phantom Design for RF-Induced Heating in MRI Scanner
4.2. Experimental Setup for RF-Induced Heating in an MRI Scanner
4.3. Synthesis and Characterization of Thermoresponsive Nanogels
4.4. Protein Encapsulation in the Thermoresponsive Nanogels
4.5. Evaluation of BSA Release using a Water Bath
4.6. RF-Induced Heating Paradigm and Release Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Besse, H.C.; Barten-van Rijbroek, A.D.; van der Wurff-Jacobs, K.M.G.; Bos, C.; Moonen, C.T.W.; Deckers, R. Tumor drug distribution after local drug delivery by hyperthermia, in vivo. Cancers 2019, 11, 1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in cancer imaging and therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Lynn, D.M.; Amiji, M.M.; Langer, R. pH-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew. Chemie Int. Ed. 2001, 40, 1707–1710. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhu, D.; Zong, S.; Yang, L.; Zhong, Y.; Cui, Y. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer–lipid bilayer. ACS Appl. Mater. Interfaces 2013, 5, 10895–10903. [Google Scholar] [CrossRef]
- Molina, M.; Giulbudagian, M.; Calderón, M. Positively charged thermoresponsive nanogels for anticancer drug delivery. Macromol. Chem. Phys. 2014, 215, 2414–2419. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef] [Green Version]
- Dunn, A.E.; Dunn, D.J.; Macmillan, A.; Whan, R.; Stait-Gardner, T.; Price, W.S.; Lim, M.; Boyer, C. Spatial and temporal control of drug release through pH and alternating magnetic field induced breakage of Schiff base bonds. Polym. Chem. 2014, 5, 3311–3315. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theune, L.E.; Charbaji, R.; Kar, M.; Wedepohl, S.; Hedtrich, S.; Calderón, M. Critical parameters for the controlled synthesis of nanogels suitable for temperature-triggered protein delivery. Mater. Sci. Eng. C 2019, 100, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly (N-vinylcaprolactam) versus poly (N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Lin, J.C. Electromagnetic Fields in Biological Systems; CRC Press: Boca Raton, FL, USA, 2011; ISBN 143985999X. [Google Scholar]
- Kok, H.P.; Crezee, J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int. J. Hyperth. 2017, 33, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Lasers as an approach for promoting drug delivery via skin. Expert Opin. Drug Deliv. 2014, 11, 599–614. [Google Scholar] [CrossRef]
- Ahmed, M.; Liu, Z.; Lukyanov, A.N.; Signoretti, S.; Horkan, C.; Monsky, W.L.; Torchilin, V.P.; Goldberg, S.N. Combination radiofrequency ablation with intratumoral liposomal doxorubicin: Effect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology 2005, 235, 469–477. [Google Scholar] [CrossRef]
- Aubry, J.-F.; Pauly, K.B.; Moonen, C.; Haar, G.; Ries, M.; Salomir, R.; Sokka, S.; Sekins, K.M.; Shapira, Y.; Ye, F. The road to clinical use of high-intensity focused ultrasound for liver cancer: Technical and clinical consensus. J. Ther. Ultrasound 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Cranston, D. A review of high intensity focused ultrasound in relation to the treatment of renal tumours and other malignancies. Ultrason. Sonochem. 2015, 27, 654–658. [Google Scholar] [CrossRef]
- Grüll, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release 2012, 161, 317–327. [Google Scholar] [CrossRef]
- May, J.P.; Li, S.-D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013, 10, 511–527. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijnen, N.; Kneepkens, E.; de Smet, M.; Langereis, S.; Heijman, E.; Grüll, H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proc. Natl. Acad. Sci. USA 2017, 114, E4802–E4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, C.; Patel, P.; Staruch, R.M.; Shaikh, S.; Nofiele, J.; Wodzak Staruch, M.; Szczepanski, D.; Williams, N.S.; Laetsch, T.; Chopra, R. Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin. Int. J. Hyperth. 2019, 36, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Staruch, R.M.; Hynynen, K.; Chopra, R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: Therapeutic effect in rabbit Vx2 tumours. Int. J. Hyperth. 2015, 31, 118–133. [Google Scholar] [CrossRef]
- Paulides, M.M.; Trefna, H.D.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency-and microwave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Gellermann, J.; Riess, H.; Wust, P. Induced hyperthermia in the reatment of cancer. In Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and supporting Measures; Springer: Dordrecht, The Netherlands, 2011; Volume 13, pp. 365–377. [Google Scholar]
- Winter, L.; Oezerdem, C.; Hoffmann, W.; van de Lindt, T.; Periquito, J.; Ji, Y.; Ghadjar, P.; Budach, V.; Wust, P.; Niendorf, T. Thermal magnetic resonance: Physics considerations and electromagnetic field simulations up to 23.5 Tesla (1GHz). Radiat. Oncol. 2015, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Winter, L.; Özerdem, C.; Hoffmann, W.; Santoro, D.; Müller, A.; Waiczies, H.; Seemann, R.; Graessl, A.; Wust, P.; Niendorf, T. Design and evaluation of a hybrid radiofrequency applicator for magnetic resonance imaging and RF induced hyperthermia: Electromagnetic field simulations up to 14.0 Tesla and proof-of-concept at 7.0 Tesla. PLoS ONE 2013, 8, e61661. [Google Scholar] [CrossRef]
- Oberacker, E.; Kuehne, A.; Nadobny, J.; Zschaeck, S.; Weihrauch, M.; Waiczies, H.; Ghadjar, P.; Wust, P.; Niendorf, T.; Winter, L. Radiofrequency applicator concepts for simultaneous MR imaging and hyperthermia treatment of glioblastoma multiforme. Curr. Dir. Biomed. Eng. 2017, 3, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, A.; Oberacker, E.; Waiczies, H.; Niendorf, T. Solving the Time-and Frequency-Multiplexed Problem of Constrained Radiofrequency Induced Hyperthermia. Cancers 2020, 12, 1072. [Google Scholar] [CrossRef]
- Eigentler, T.W.; Winter, L.; Han, H.; Oberacker, E.; Kuehne, A.; Waiczies, H.; Schmitter, S.; Boehmert, L.; Prinz, C.; Trefna, H.D. Wideband Self-Grounded Bow-Tie Antenna for Thermal MR. NMR Biomed. 2020, 33, e4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miceli, E.; Kuropka, B.; Rosenauer, C.; Osorio Blanco, E.R.; Theune, L.E.; Kar, M.; Weise, C.; Morsbach, S.; Freund, C.; Calderón, M. Understanding the elusive protein corona of thermoresponsive nanogels. Nanomedicine 2018, 13, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Plank, R.; Yealland, G.; Miceli, E.; Cunha, D.L.; Graff, P.; Thomforde, S.; Gruber, R.; Moosbrugger-Martinz, V.; Eckl, K.; Calderón, M. Transglutaminase 1 Replacement Therapy Successfully Mitigates the Autosomal Recessive Congenital Ichthyosis Phenotype in Full-Thickness Skin Disease Equivalents. J. Investig. Dermatol. 2019, 139, 1191–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulbudagian, M.; Yealland, G.; Hönzke, S.; Edlich, A.; Geisendörfer, B.; Kleuser, B.; Hedtrich, S.; Calderón, M. Breaking the barrier-potent anti-inflammatory activity following efficient topical delivery of etanercept using thermoresponsive nanogels. Theranostics 2018, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- De León, A.S.; Molina, M.; Wedepohl, S.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J.; Calderón, M. Immobilization of stimuli-responsive nanogels onto honeycomb porous surfaces and controlled release of proteins. Langmuir 2016, 32, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderón, M.; Frieß, W.; Hedtrich, S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1179–1187. [Google Scholar] [CrossRef]
- Landon, C.D.; Park, J.-Y.; Needham, D.; Dewhirst, M.W. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011, 3, 38. [Google Scholar] [CrossRef]
- De Smet, M.; Langereis, S.; van den Bosch, S.; Grüll, H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J. Control. Release 2010, 143, 120–127. [Google Scholar] [CrossRef]
- Niendorf, T.; Sodickson, D.K. Parallel imaging in cardiovascular MRI: Methods and applications. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2006, 19, 325–341. [Google Scholar] [CrossRef]
- Sodickson, D.K.; Hardy, C.J.; Zhu, Y.; Giaquinto, R.O.; Gross, P.; Kenwood, G.; Niendorf, T.; Lejay, H.; McKenzie, C.A.; Ohliger, M.A. Rapid Volumetric MRI Using Parallel Imaging With Order-of-Magnitude Accelerations and a 32-Element RF Coil Array: Feasibility and implications1. Acad. Radiol. 2005, 12, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, K.; Hezel, F.; Klix, S.; Mekle, R.; Wuerfel, J.; Niendorf, T. Simultaneous dual contrast weighting using double echo rapid acquisition with relaxation enhancement (RARE) imaging. Magn. Reson. Med. 2014, 72, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Huelnhagen, T.; Oberacker, E.; Wenz, D.; Kuehne, A.; Waiczies, H.; Schmitter, S.; Stachs, O.; Niendorf, T. Multiband diffusion-weighted MRI of the eye and orbit free of geometric distortions using a RARE-EPI hybrid. NMR Biomed. 2018, 31, e3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Waiczies, H.; Winter, L.; Neumanova, P.; Hofmann, D.; Rieger, J.; Mekle, R.; Waiczies, S.; Niendorf, T. Eight-channel transceiver RF coil array tailored for 1H/19F MR of the human knee and fluorinated drugs at 7.0 T. NMR Biomed. 2015, 28, 726–737. [Google Scholar] [CrossRef] [Green Version]
- Prinz, C.; Delgado, P.R.; Eigentler, T.W.; Starke, L.; Niendorf, T.; Waiczies, S. Toward 19 F magnetic resonance thermometry: Spin–lattice and spin–spin-relaxation times and temperature dependence of fluorinated drugs at 9.4 T. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Theune, L.E.; Calderón, M. Effect of crosslinking density on thermoresponsive nanogels: A study on the size control and the kinetics release of biomacromolecules. Eur. Polym. J. 2020, 124, 109478. [Google Scholar] [CrossRef]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin loaded dual pH-and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peller, M.; Willerding, L.; Limmer, S.; Hossann, M.; Dietrich, O.; Ingrisch, M.; Sroka, R.; Lindner, L.H. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J. Control. Release 2016, 237, 138–146. [Google Scholar] [CrossRef]
- Edlich, A.; Gerecke, C.; Giulbudagian, M.; Neumann, F.; Hedtrich, S.; Schäfer-Korting, M.; Ma, N.; Calderon, M.; Kleuser, B. Specific uptake mechanisms of well-tolerated thermoresponsive polyglycerol-based nanogels in antigen-presenting cells of the skin. Eur. J. Pharm. Biopharm. 2017, 116, 155–163. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.-C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.-M. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306. [Google Scholar] [CrossRef] [PubMed]
- Rimondino, G.N.; Miceli, E.; Molina, M.; Wedepohl, S.; Thierbach, S.; Rühl, E.; Strumia, M.; Martinelli, M.; Calderón, M. Rational design of dendritic thermoresponsive nanogels that undergo phase transition under endolysosomal conditions. J. Mater. Chem. B 2017, 5, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Willerding, L.; Limmer, S.; Hossann, M.; Zengerle, A.; Wachholz, K.; ten Hagen, T.L.M.; Koning, G.A.; Sroka, R.; Lindner, L.H.; Peller, M. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J. Control. Release 2016, 222, 47–55. [Google Scholar] [CrossRef]
- Trattnig, S.; Springer, E.; Bogner, W.; Hangel, G.; Strasser, B.; Dymerska, B.; Cardoso, P.L.; Robinson, S.D. Key clinical benefits of neuroimaging at 7 T. Neuroimage 2018, 168, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Obusez, E.C.; Lowe, M.; Oh, S.-H.; Wang, I.; Bullen, J.; Ruggieri, P.; Hill, V.; Lockwood, D.; Emch, T.; Moon, D. 7T MR of intracranial pathology: Preliminary observations and comparisons to 3T and 1.5 T. Neuroimage 2018, 168, 459–476. [Google Scholar] [CrossRef]
- De Cocker, L.J.L.; Lindenholz, A.; Zwanenburg, J.J.M.; van der Kolk, A.G.; Zwartbol, M.; Luijten, P.R.; Hendrikse, J. Clinical vascular imaging in the brain at 7 T. Neuroimage 2018, 168, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niendorf, T.; Schulz-Menger, J.; Paul, K.; Huelnhagen, T.; Ferrari, V.A.; Hodge, R. High field cardiac magnetic resonance imaging: A case for ultrahigh field cardiac magnetic resonance. Circ. Cardiovasc. Imaging 2017, 10, e005460. [Google Scholar] [CrossRef] [Green Version]
- Klix, S.; Els, A.; Paul, K.; Graessl, A.; Oezerdem, C.; Weinberger, O.; Winter, L.; Thalhammer, C.; Huelnhagen, T.; Rieger, J. On the subjective acceptance during cardiovascular magnetic resonance imaging at 7.0 Tesla. J. Cardiovasc. Magn. Reson. 2015, 17, P13. [Google Scholar] [CrossRef] [Green Version]
- Versluis, M.J.; Teeuwisse, W.M.; Kan, H.E.; van Buchem, M.A.; Webb, A.G.; van Osch, M.J. Subject tolerance of 7 T MRI examinations. J. Magn. Reson. Imaging 2013, 38, 722–725. [Google Scholar] [CrossRef]
- Rauschenberg, J.; Nagel, A.M.; Ladd, S.C.; Theysohn, J.M.; Ladd, M.E.; Möller, H.E.; Trampel, R.; Turner, R.; Pohmann, R.; Scheffler, K. Multicenter study of subjective acceptance during magnetic resonance imaging at 7 and 9.4 T. Invest. Radiol. 2014, 49, 249–259. [Google Scholar] [CrossRef]
- Winter, L.; Niendorf, T. Electrodynamics and radiofrequency antenna concepts for human magnetic resonance at 23.5 T (1 GHz) and beyond. Magn. Reson. Mater. Physics, Biol. Med. 2016, 29, 641–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Hoffmann, W.; Pham, M.; Dunn, A.E.; Han, H.; Özerdem, C.; Waiczies, H.; Rohloff, M.; Endemann, B.; Boyer, C.; et al. High peak and high average radiofrequency power transmit/receive switch for thermal magnetic resonance. Magn. Reson. Med. 2018, 80, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, J.C.; Strumia, M.C.; Welker, P.; Licha, K.; Steinhilber, D.; Mutihac, R.-C.; Calderón, M. Thermosensitive nanogels based on dendritic polyglycerol and N-isopropylacrylamide for biomedical applications. Soft Matter 2011, 7, 11259–11266. [Google Scholar] [CrossRef]
- Ishihara, Y.; Calderon, A.; Watanabe, H.; Okamoto, K.; Suzuki, Y.; Kuroda, K.; Suzuki, Y. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 1995, 34, 814–823. [Google Scholar] [CrossRef]
- Rieke, V.; Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Wonneberger, U.; Schnackenburg, B.; Wlodarczyk, W.; Walter, T.; Streitparth, F.; Rump, J.; Teichgräber, U.K.M. Intradiscal temperature monitoring using double gradient-echo pulse sequences at 1.0 T. J. Magn. Reson. Imaging 2010, 31, 1499–1503. [Google Scholar] [CrossRef]
- Fuchs, V.R.; Sox Jr, H.C. Physicians’ views of the relative importance of thirty medical innovations. Health Aff. 2001, 20, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Vyas, K. 10 Medical Inventions of All Time That Changed the World. Available online: https://interestingengineering.com/10-medical-inventions-of-all-time-that-changed-the-world (accessed on 29 August 2019).








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Winter, L.; Navarro, L.; Ku, M.-C.; Periquito, J.S.; Pham, M.; Hoffmann, W.; Theune, L.E.; Calderón, M.; Niendorf, T. Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study. Cancers 2020, 12, 1380. https://doi.org/10.3390/cancers12061380
Ji Y, Winter L, Navarro L, Ku M-C, Periquito JS, Pham M, Hoffmann W, Theune LE, Calderón M, Niendorf T. Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study. Cancers. 2020; 12(6):1380. https://doi.org/10.3390/cancers12061380
Chicago/Turabian StyleJi, Yiyi, Lukas Winter, Lucila Navarro, Min-Chi Ku, João S. Periquito, Michal Pham, Werner Hoffmann, Loryn E. Theune, Marcelo Calderón, and Thoralf Niendorf. 2020. "Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study" Cancers 12, no. 6: 1380. https://doi.org/10.3390/cancers12061380
APA StyleJi, Y., Winter, L., Navarro, L., Ku, M. -C., Periquito, J. S., Pham, M., Hoffmann, W., Theune, L. E., Calderón, M., & Niendorf, T. (2020). Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study. Cancers, 12(6), 1380. https://doi.org/10.3390/cancers12061380