Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies
Abstract
:1. Introduction
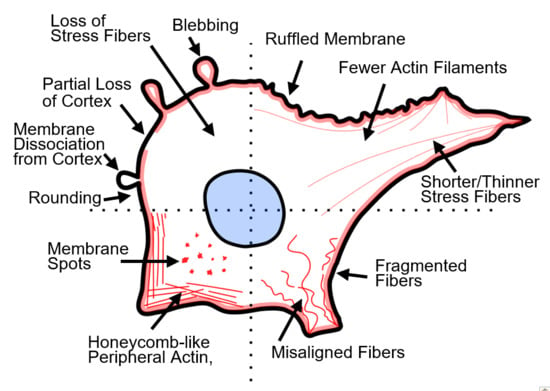
2. Actin and PEF-induced Actin Disruption
2.1. Actin Disruption
2.1.1. Actin-Induced Cell Elasticity Changes
2.1.2. Actin, Membrane Permeability, and Cell Viability
3. Microtubules and PEF-Induced Microtubule Disruption
3.1. Microtubules Disruption
3.2. Microtubules, Membrane Permeability, and Cell Viability
4. Intermediate Filaments and Septins
5. Mechanisms of Cytoskeletal Disruption
5.1. Actin—Direct Mechanisms
5.2. Microtubules—Direct Mechanisms
5.3. Swellling/Volume Change
5.4. Cytosolic Calcium Concentration
5.5. ATP Depletion
5.6. Additional Mechanisms
5.7. Disruption Mechanisms and Pulse Length
6. Cell-Matrix and Cell–Cell Junction Disruption
6.1. Cell-Matrix Disruption
6.2. Cell-Cell Junction Disruption
7. Considerations for Electroporation Therapies
Cytoskeletal Targets for Improved PEF Therapies
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| ATP | Adenosine Triphosphate |
| COL | colchicine |
| CytB | cytochalasin B |
| CytD | cytochalasin D |
| ECT | electrochemotherapy |
| ER | endoplasmic reticulum |
| freq | frequency |
| GET | gene electrotransfer |
| GTP | Guanosine Triphosphate |
| GUV | giant unilamellar vesicle |
| IF | intermediate filaments |
| IRE | irreversible electroporation |
| JAS | jasplakinolide |
| LatA | latrunculin A |
| LatB | latrunculin B |
| MD | molecular dynamics |
| MT | microtubules |
| NOC | nocodazole |
| PEFs | pulsed electric fields |
| PHD | phalloidin |
| PI | propidium iodide |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PLC | phospholipase C |
| pMLC | phosphorylated myosin light chain |
| PTX | paclitaxel |
| p | pulses |
| YM | Young’s modulus |
| Cell Types | |
| B16-F10 | mouse melanoma |
| C2C12 | mouse myoblasts |
| CHO | Chinese hamster ovary cells (CHO wild type, CHO-K1, CHO-WTT clone) |
| HeLa | human cervical cancer (adenocarcinoma) |
| HepG2 | human hepatocellular carcinoma |
| HMEC-1 | human dermal microvascular endothelial cells |
| Jurkat | Clone E6-1 human T lymphocytes |
| MCF-7 | human breast cancer (adenocarcinoma) |
| MDA-MB-231 | human breast cancer (adenocarcinoma) |
| MRC-5 | human lung fibroblasts |
| NCI-H460 | human lung carcinoma |
| NIH/3T3 | mouse fibroblasts |
| RBL-2H3 | rat basophilic cells |
| RD | human rhabdomyosarcoma |
| SV40 | immortalized fibroblasts |
| U-87 MG | human glioblastoma |
| U-937 | human monocytes |
| WB-F344 | rat liver epithelial cells. |
References
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue Ablation with Irreversible Electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Sano, M.; Rossmeisl, J.H.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 2011, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, H.; Nielsen, K.; De Jong, M.C.; Van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.; Meijer, S.; Van Kuijk, C.; Tol, P.M.; Meijerink, M.R. Irreversible Electroporation for Nonthermal Tumor Ablation in the Clinical Setting: A Systematic Review of Safety and Efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Yarmush, M.L. Nonthermal Irreversible Electroporation: Fundamentals, Applications, and Challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Davalos, R.V.; Rubinsky, B.; Mir, L.M. Theoretical analysis of the thermal effects during in vivo tissue electroporation. Bioelectrochemistry 2003, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2013, 39, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Pliquett, U.; Chen, X.; Ford, W.; Swanson, R.J.; Beebe, S.J.; Kolb, J.F.; Schoenbach, K.H. Nanosecond pulsed electric fields cause melanomas to self-destruct. Biochem. Biophys. Res. Commun. 2006, 343, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Willis, L.K.; Schoenbach, K.H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003, 17, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Somiari, S.; Glasspool-Malone, J.; Drabick, J.J.; Gilbert, R.A.; Heller, R.; Jaroszeski, M.J.; Malone, R.W. Theory and in Vivo Application of Electroporative Gene Delivery. Mol. Ther. 2000, 2, 178–187. [Google Scholar] [CrossRef]
- Heller, L.; Pottinger, C.; Jaroszeski, M.J.; Gilbert, R.; Heller, R. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000, 10, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sales, N.S.; Silva, J.R.; Aps, L.R.; Silva, M.O.; Porchia, B.F.; Ferreira, L.C.S.; Diniz, M.O. In vivo electroporation enhances vaccine-mediated therapeutic control of human papilloma virus-associated tumors by the activation of multifunctional and effector memory CD8+ T cells. Vaccine 2017, 35, 7240–7249. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, J.; Joo, S.W.; Banerjee, A.N.; Qian, S. Cell electrofusion in microfluidic devices: A review. Sens. Actuators B Chem. 2013, 178, 63–85. [Google Scholar] [CrossRef]
- Chiu, F.W.Y.; Bagci, H.; Fisher, A.G.; Demello, A.J.; Elvira, K. A microfluidic toolbox for cell fusion. J. Chem. Technol. Biotechnol. 2015, 91, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Rowan, N.J.; MacGregor, S.; Anderson, J.; Fouracre, R.; Farish, O. Pulsed electric field inactivation of diarrhoeagenic Bacillus cereus through irreversible electroporation. Lett. Appl. Microbiol. 2000, 31, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Novickij, V.; Stanevičienė, R.; Grainys, A.; Luksa, J.; Badokas, K.; Krivorotova, T.; Sereikaite, J.; Novickij, J.; Servienė, E. Electroporation-assisted inactivation of Escherichia coli using nisin-loaded pectin nanoparticles. Innov. Food Sci. Emerg. Technol. 2016, 38, 98–104. [Google Scholar] [CrossRef]
- Sano, M.; Neal, R.E.; Garcia, P.A.; Gerber, D.A.; Robertson, J.; Davalos, R.V. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed. Eng. Online 2010, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Maor, E.; Rubinsky, B. Nonthermal Irreversible Electroporation for Tissue Decellularization. J. Biomech. Eng. 2010, 132, 091003. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavcic, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Sack, M.; Sigler, J.; Frenzel, S.; Eing, C.; Arnold, J.; Michelberger, T.; Frey, W.; Attmann, F.; Stukenbrock, L.; Müller, G. Research on Industrial-Scale Electroporation Devices Fostering the Extraction of Substances from Biological Tissue. Food Eng. Rev. 2010, 2, 147–156. [Google Scholar] [CrossRef]
- Chu, G.; Berg, P.; Hayakawa, H. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987, 15, 1311–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukany, P.E.; Morss, A.; Liao, W.-C.; Henslee, B.; Jung, H.; Zhang, X.; Yu, B.; Wang, X.; Wu, Y.; Li, L.; et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotechnol. 2011, 6, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Zhan, Y.; Wang, J.; Lu, C. Transfection of cells using flow-through electroporation based on constant voltage. Nat. Protoc. 2011, 6, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Nanki, K.; Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 2015, 10, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Bestman, J.E.; Ewald, R.C.; Chiu, S.-L.; Cline, H.T. In vivo single-cell electroporation for transfer of DNA and macromolecules. Nat. Protoc. 2006, 1, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Potter, H.; Heller, R. Transfection by Electroporation. Curr. Protoc. Mol. Boil. 2018, 121, 9.3.1–9.3.13. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavcic, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. IEEE Trans. Biomed. Eng. 2014, 62, 4–20. [Google Scholar] [CrossRef]
- Chopinet, L.; Rols, M.-P. Nanosecond electric pulses: A mini-review of the present state of the art. Bioelectrochemistry 2015, 103, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; Tol, P.M.; Davalos, R.V.; Rubinsky, B.; De Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Cemazar, M. Effects of Electroporation of Mammalian Cells on Cytoskeleton and Intercellular Connections. In Handbook of Electroporation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 307–321. [Google Scholar]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Boil. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Svitkina, T.M. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Boil. 2018, 10, a018267. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.; Mitchison, T.J. Microtubule Polymerization Dynamics. Annu. Rev. Cell Dev. Boil. 1997, 13, 83–117. [Google Scholar] [CrossRef] [Green Version]
- Son, R.S.; Smith, K.C.; Gowrishankar, T.R.; Vernier, P.T.; Weaver, J.C. Basic features of a cell electroporation model: Illustrative behavior for two very different pulses. J. Membr. Boil. 2014, 247, 1209–1228. [Google Scholar] [CrossRef] [Green Version]
- Napotnik, T.B.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavcic, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Ziegler, M.J.; Sun, Y.; Gundersen, M.A.; Tieleman, D.P. Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers—In cells andin silico. Phys. Boil. 2006, 3, 233–247. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Craft, C.M.; Gundersen, M.A. Nanoelectropulse-Induced Phosphatidylserine Translocation. Biophys. J. 2004, 86, 4040–4048. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; White, J.; Blackmore, P.F.; Deng, Y.; Somers, K.; Schoenbach, K.H. Diverse Effects of Nanosecond Pulsed Electric Fields on Cells and Tissues. DNA Cell Boil. 2003, 22, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1828, 981–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napotnik, T.B.; Gundersen, M.A.; Miklavcic, D.; Vernier, P.T.; Wu, Y.-H. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics 2011, 33, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.J.; Chen, Y.-J.; Sain, N.M.; Schoenbach, K.H.; Xiao, S. Transient Features in Nanosecond Pulsed Electric Fields Differentially Modulate Mitochondria and Viability. PLoS ONE 2012, 7, e51349. [Google Scholar] [CrossRef]
- Carr, L.; Bardet, S.M.; Burke, R.C.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Calcium-independent disruption of microtubule dynamics by nanosecond pulsed electric fields in U87 human glioblastoma cells. Sci. Rep. 2017, 7, 41267. [Google Scholar] [CrossRef]
- Stacey, M.; Stickley, J.; Fox, P.; Statler, V.; Schoenbach, K.; Beebe, S.J.; Buescher, S. Differential effects in cells exposed to ultra-short, high intensity electric fields: Cell survival, DNA damage, and cell cycle analysis. Mutat. Res. Mol. Mech. Mutagen. 2003, 542, 65–75. [Google Scholar] [CrossRef]
- Stacey, M.; Fox, P.; Buescher, S.; Kolb, J.F. Nanosecond pulsed electric field induced cytoskeleton, nuclear membrane and telomere damage adversely impact cell survival. Bioelectrochemistry 2011, 82, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Nesin, V.; Pakhomov, A. Inhibition of voltage-gated Na(+) current by nanosecond pulsed electric field (nsPEF) is not mediated by Na(+) influx or Ca(2+) signaling. Bioelectromagnetics 2012, 33, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Ford, W.E.; Ren, W.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Nanosecond pulsed electric fields stimulate apoptosis without release of pro-apoptotic factors from mitochondria in B16f10 melanoma. Arch. Biochem. Biophys. 2010, 497, 82–89. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Boil. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meulenberg, C.J.W.; Todorovic, V.; Čemažar, M. Differential Cellular Effects of Electroporation and Electrochemotherapy in Monolayers of Human Microvascular Endothelial Cells. PLoS ONE 2012, 7, e52713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, G.L.; Roth, C.; Tolstykh, G.; Kuipers, M.; Ibey, B.L. Disruption of the actin cortex contributes to susceptibility of mammalian cells to nanosecond pulsed electric fields. Bioelectromagnetics 2014, 35, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Steuer, A.; Wende, K.; Babica, P.; Kolb, J.F. Elasticity and tumorigenic characteristics of cells in a monolayer after nanosecond pulsed electric field exposure. Eur. Biophys. J. 2017, 6, 1565–1580. [Google Scholar] [CrossRef]
- Pehlivanova, V.N.; Tsoneva, I.; Tzoneva, R.D. Multiple effects of electroporation on the adhesive behaviour of breast cancer cells and fibroblasts. Cancer Cell Int. 2012, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Steuer, A.; Schmidt, A.; Laboha, P.; Babica, P.; Kolb, J.F.; Macikova, P. Transient suppression of gap junctional intercellular communication after exposure to 100-nanosecond pulsed electric fields. Bioelectrochemistry 2016, 112, 33–46. [Google Scholar] [CrossRef]
- Berghöfer, T.; Eing, C.; Flickinger, B.; Hohenberger, P.; Wegner, L.; Frey, W.; Nick, P. Nanosecond electric pulses trigger actin responses in plant cells. Biochem. Biophys. Res. Commun. 2009, 387, 590–595. [Google Scholar] [CrossRef]
- Chopinet, L.; Roduit, C.; Rols, M.-P.; Dague, E. Destabilization induced by electropermeabilization analyzed by atomic force microscopy. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2223–2229. [Google Scholar] [CrossRef] [Green Version]
- Kanthou, C.; Brezar, S.K.; Sersa, G.; Tozer, G.; Zupanic, A.; Cemazar, M. The endothelial cytoskeleton as a target of electroporation-based therapies. Mol. Cancer Ther. 2006, 5, 3145–3152. [Google Scholar] [CrossRef] [Green Version]
- Rols, M.-P.; Teissie, J. Experimental evidence for the involvement of the cytoskeleton in mammalian cell electropermeabilization. Biochim. Biophys. Acta (BBA) Biomembr. 1992, 1111, 45–50. [Google Scholar] [CrossRef]
- Teissie, J.; Rols, M.-P. Manipulation of Cell Cytoskeleton Affects the Lifetime of Cell Membrane Electropermeabilization. Ann. N. Y. Acad. Sci. 1994, 720, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Rols, M.P.; Teissie, J. Evidence for cytoskeleton implication in cell electropermeabilization and electrofusion. In Aip Conference Proceedings; AIP Publishing: College Park, MD, USA, 1991; Volume 226, pp. 251–266. [Google Scholar]
- Dutta, D.; Asmar, A.; Stacey, M. Effects of nanosecond pulse electric fields on cellular elasticity. Micron 2015, 72, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, A.; Xiao, S.; Pakhomova, O.N.; Semenov, I.; Kuipers, M.A.; Ibey, B.L. Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry 2014, 100, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yizraeli, M.L.; Weihs, D. Time-Dependent Micromechanical Responses of Breast Cancer Cells and Adjacent Fibroblasts to Electric Treatment. Cell Biophys. 2011, 61, 605–618. [Google Scholar] [CrossRef]
- Rassokhin, M.A.; Pakhomov, A.G. Electric field exposure triggers and guides formation of pseudopod-like blebs in U937 monocytes. J. Membr. Boil. 2012, 245, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Harkin, D.G.; Hay, E.D. Effects of electroporation on the tubulin cytoskeleton and directed migration of corneal fibroblasts cultured within collagen matrices. Cell Motil. Cytoskelet. 1996, 35, 345–357. [Google Scholar] [CrossRef]
- Louise, C.; Etienne, D.; Marie-Pierre, R. AFM sensing cortical actin cytoskeleton destabilization during plasma membrane electropermeabilization. Cytoskeleton 2014, 71, 587–594. [Google Scholar] [CrossRef]
- Hohenberger, P.; Eing, C.; Straessner, R.; Durst, S.; Frey, W.; Nick, P. Plant actin controls membrane permeability. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 2304–2312. [Google Scholar] [CrossRef] [Green Version]
- Downey, G. Actin assembly in electropermeabilized neutrophils: Role of intracellular calcium. J. Cell Boil. 1990, 110, 1975–1982. [Google Scholar] [CrossRef]
- Perrier, D.L.; Vahid, A.; Kathavi, V.; Stam, L.; Rems, L.; Mulla, Y.; Muralidharan, A.; Koenderink, G.H.; Kreutzer, M.T.; Boukany, P.E. Response of an actin network in vesicles under electric pulses. Sci. Rep. 2019, 9, 8151. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, A.; Gehl, J.; Daczewska, M.; Saczko, J.; Frandsen, S.K.; Kulbacka, J. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget 2018, 9, 11604–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.B.; Lee, S.; Chung, J.H.; Kim, S.N.; Sung, C.K.; Baik, K.Y. Effects of Actin Cytoskeleton Disruption on Electroporation in Vitro. Appl. Biochem. Biotechnol. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Roth, C.C.; Dalzell, D.R.; Kuipers, M.; Ibey, B.L. Calcium influx affects intracellular transport and membrane repair following nanosecond pulsed electric field exposure. J. Biomed. Opt. 2014, 19, 55005. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Roth, C.C.; Kuipers, M.A.; Tolstykh, G.P.; Beier, H.; Ibey, B.L. Permeabilization of the nuclear envelope following nanosecond pulsed electric field exposure. Biochem. Biophys. Res. Commun. 2016, 470, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolstykh, G.P.; Thompson, G.L.; Beier, H.T.; Steelman, Z.A.; Ibey, B.L. nsPEF-induced PIP2 depletion, PLC activity and actin cytoskeletal cortex remodeling are responsible for post-exposure cellular swelling and blebbing. Biochem. Biophys. Rep. 2017, 9, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Tang, L.; Zeng, C.; Wang, J.; Luo, X.; Yao, C.; Sun, C. Effect of actin cytoskeleton disruption on electric pulse-induced apoptosis and electroporation in tumour cells. Cell Boil. Int. 2011, 35, 99–104. [Google Scholar] [CrossRef]
- Marracino, P.; Havelka, D.; Průša, J.; Liberti, M.; Tuszynski, J.A.; Ayoub, A.T.; Apollonio, F.; Cifra, M. Tubulin response to intense nanosecond-scale electric field in molecular dynamics simulation. Sci. Rep. 2019, 9, 10477. [Google Scholar] [CrossRef]
- Průša, J.; Cifra, M. Molecular dynamics simulation of the nanosecond pulsed electric field effect on kinesin nanomotor. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chafai, D.E.; Sulimenko, V.; Havelka, D.; Kubínová, L.; Dráber, P.; Cifra, M. Reversible and Irreversible Modulation of Tubulin Self-Assembly by Intense Nanosecond Pulsed Electric Fields. Adv. Mater. 2019, 31, e1903636. [Google Scholar] [CrossRef] [Green Version]
- Havelka, D.; Chafai, D.E.; Krivosudský, O.; Klebanovych, A.; Vostárek, F.; Kubínová, L.; Dráber, P.; Cifra, M. Nanosecond Pulsed Electric Field Lab-on-Chip Integrated in Super-Resolution Microscope for Cytoskeleton Imaging. Adv. Mater. Technol. 2019, 5. [Google Scholar] [CrossRef]
- Thompson, G.L.; Roth, C.; Tolstykh, G.; Kuipers, M.; Ibey, B.L. Role of Cytoskeleton and Elastic Moduli in Cellular Response to Nanosecond Pulsed Electric Fields. In Terahertz and Ultrashort Electromagnetic Pulses for Biomedical Applications; Wilmink, G.J., Ibey, B.L., Eds.; SPIE Press: Bellingham, WA, USA, 2013; Volume 8585. [Google Scholar]
- Timmons, J.J.; Preto, J.; Tuszynski, J.A.; Wong, E.T. Tubulin’s response to external electric fields by molecular dynamics simulations. PLoS ONE 2018, 13, e0202141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef] [Green Version]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthakrishnan, R.; Guck, J.; Wottawah, F.; Schinkinger, S.; Lincoln, B.; Romeyke, M.; Moon, T.; Käs, J. Quantifying the contribution of actin networks to the elastic strength of fibroblasts. J. Theor. Boil. 2006, 242, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Krassen, H.; Pliquett, U.; Neumann, E. Nonlinear current–voltage relationship of the plasma membrane of single CHO cells. Bioelectrochemistry 2007, 70, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, M.; Miklavcic, D. Theoretical and experimental analysis of conductivity, ion diffusion and molecular transport during cell electroporation — Relation between short-lived and long-lived pores. Bioelectrochemistry 2008, 74, 38–46. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Kolb, J.F.; White, J.A.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 2007, 28, 655–663. [Google Scholar] [CrossRef]
- Bier, M.; Hammer, S.M.; Canaday, D.J.; Lee, R.C. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 1999, 20, 194–201. [Google Scholar] [CrossRef]
- Tarek, M. Membrane Electroporation: A Molecular Dynamics Simulation. Biophys. J. 2005, 88, 4045–4053. [Google Scholar] [CrossRef] [Green Version]
- Levine, Z.A.; Vernier, P.T. Life Cycle of an Electropore: Field-Dependent and Field-Independent Steps in Pore Creation and Annihilation. J. Membr. Boil. 2010, 236, 27–36. [Google Scholar] [CrossRef]
- Bennett, D.; Sapay, N.; Tieleman, D.P. Atomistic Simulations of Pore Formation and Closure in Lipid Bilayers. Biophys. J. 2014, 106, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.C.; Vernier, P.T. Pore lifetimes in cell electroporation: Complex dark pores? arXiv preprint 2017, arXiv:1708.07478. Available online: https://arxiv.org/abs/1708.07478 (accessed on 31 January 2020).
- Dimova, R.; Riske, K.A. Electrodeformation, Electroporation, and Electrofusion of Giant Unilamellar Vesicles. In Handbook Electroporation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 235–252. [Google Scholar]
- Sözer, E.B.; Haldar, S.; Blank, P.S.; Castellani, F.; Vernier, P.T.; Zimmerberg, J. Ultra-Fast Electroporation of Giant Unilamellar Vesicles—Experimental Validation of a Molecular Model; BioRxiv: Cold Spring Harbor, NY, USA, 2020. [Google Scholar]
- Lira, R.B.; Dimova, R.; Riske, K.A. Giant Unilamellar Vesicles Formed by Hybrid Films of Agarose and Lipids Display Altered Mechanical Properties. Biophys. J. 2014, 107, 1609–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowers, A. The long-lived fusogenic state induced in erythrocyte ghosts by electric pulses is not laterally mobile. Biophys. J. 1987, 52, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Boil. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Boil. 1993, 120, 923–934. [Google Scholar] [CrossRef]
- Kasas, S.; Dietler, G. Techniques for Measuring Microtubule Stiffness. Curr. Nanosci. 2007, 3, 79–96. [Google Scholar] [CrossRef]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Boil. 2011, 12, 709–721. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Gardner, M.K.; Zanic, M.; Howard, J. Microtubule catastrophe and rescue. Curr. Opin. Cell Boil. 2012, 25, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Bodakuntla, S.; Jijumon, A.; Villablanca, C.; Gonzalez-Billault, C.; Janke, C. Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Boil. 2019, 29, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Miyakawa, T.; Tanokura, M. Overview of the mechanism of cytoskeletal motors based on structure. Biophys. Rev. 2017, 10, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N. Kinesin and Dynein Superfamily Proteins and the Mechanism of Organelle Transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu. Rev. Cell Dev. Boil. 2018, 34, 1–28. [Google Scholar] [CrossRef]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Boil. 2017, 5, 81. [Google Scholar] [CrossRef]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Boil. 2012, 13, 183–194. [Google Scholar] [CrossRef]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor Treating Fields Perturb the Localization of Septins and Cause Aberrant Mitotic Exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef] [Green Version]
- Riske, K.A.; Dimova, R. Electro-Deformation and Poration of Giant Vesicles Viewed with High Temporal Resolution. Biophys. J. 2004, 88, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Bashirzadeh, Y.; Liu, A.P. Encapsulation of the cytoskeleton: Towards mimicking the mechanics of a cell. Soft Matter 2019, 15, 8425–8436. [Google Scholar] [CrossRef]
- Kim, T.; Kao, M.-T.; Hasselbrink, E.F.; Meyhofer, E. Active Alignment of Microtubules with Electric Fields. Nano Lett. 2007, 7, 211–217. [Google Scholar] [CrossRef]
- Kim, T.; Kao, M.-T.; Hasselbrink, E.F.; Meyhofer, E. Nanomechanical Model of Microtubule Translocation in the Presence of Electric Fields. Biophys. J. 2008, 94, 3880–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, K.J.; Mavromatos, N.E.; Michette, A.; Stracke, R.; Unger, E. Movement and Alignment of Microtubules in Electric Fields and Electric-Dipole-Moment Estimates. Electromagn. Boil. Med. 2005, 24, 319–330. [Google Scholar] [CrossRef]
- Minoura, I.; Muto, E. Dielectric Measurement of Individual Microtubules Using the Electroorientation Method. Biophys. J. 2006, 90, 3739–3748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, R.; Soares, M.H.A.; Melo, L. Microtubule behavior under strong electromagnetic fields. Mater. Sci. Eng. C 2007, 27, 1207–1210. [Google Scholar] [CrossRef]
- Stracke, R.; Böhm, K.; Wollweber, L.; Tuszynski, J.; Unger, E. Analysis of the migration behaviour of single microtubules in electric fields. Biochem. Biophys. Res. Commun. 2002, 293, 602–609. [Google Scholar] [CrossRef]
- Heuvel, M.G.L.V.D.; Bondesan, R.; Lagomarsino, M.C.; Dekker, C. Single-Molecule Observation of Anomalous Electrohydrodynamic Orientation of Microtubules. Phys. Rev. Lett. 2008, 101, 118301. [Google Scholar] [CrossRef] [Green Version]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [Green Version]
- Setayandeh, S.S.; Lohrasebi, A. Influence of GHz electric fields on the mechanical properties of a microtubule. J. Mol. Model. 2015, 21, 85. [Google Scholar] [CrossRef]
- Setayandeh, S.S.; Lohrasebi, A. The effects of external electric fields of 900 MHz and 2450 MHz frequencies on αβ-tubulin dimer stabilized by paclitaxel: Molecular dynamics approach. J. Theor. Comput. Chem. 2016, 15, 1650010. [Google Scholar] [CrossRef]
- Saeidi, H.R.; Setayandeh, S.S.; Lohrasebi, A. Molecular modeling of oscillating GHz electric field influence on the kinesin affinity to microtubule. Chin. Phys. B 2015, 24, 080701. [Google Scholar] [CrossRef]
- Roth, C.; Barnes, R.A.; Ibey, B.L.; Beier, H.; Mimun, L.C.; Maswadi, S.M.; Shadaram, M.; Glickman, R.D. Characterization of Pressure Transients Generated by Nanosecond Electrical Pulse (nsEP) Exposure. Sci. Rep. 2015, 5, 15063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrazdira, I.; Škorpíková, J.; Dolníková, M. Ultrasonically induced alterations of cultured tumour cells. Eur. J. Ultrasound 1998, 8, 43–49. [Google Scholar] [CrossRef]
- Skorpíková, J.; Dolníková, M.; Hrazdira, I.; Janisch, R. Changes in microtubules and microfilaments due to a combined effect of ultrasound and cytostatics in HeLa cells. Folia Boil. 2001, 47, 143–147. [Google Scholar]
- Henson, J.H. Relationships between the actin cytoskeleton and cell volume regulation. Microsc. Res. Tech. 1999, 47, 155–162. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Jaepel, J.; Blum, R. Capturing ER calcium dynamics. Eur. J. Cell Boil. 2011, 90, 613–619. [Google Scholar] [CrossRef]
- O’Brien, E.T.; Salmon, E.; Erickson, H.P. How calcium causes microtubule depolymerization. Cell Motil. Cytoskelet. 1997, 36, 125–135. [Google Scholar] [CrossRef]
- Keith, C.; DiPaola, M.; Maxfield, F.R.; Shelanski, M.L. Microinjection of Ca++-Calmodulin Causes a Localized Depolymerization of Microtubules. J. Cell Biol. 1983, 97, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.-C.; Kuo, G.-H.; Chang, S.-W.; Tsai, P.-J. Ca2+ Signaling in Cytoskeletal Reorganization, Cell Migration, and Cancer Metastasis. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Titushkin, I.; Cho, M.R. Regulation of Cell Cytoskeleton and Membrane Mechanics by Electric Field: Role of Linker Proteins. Biophys. J. 2009, 96, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Gissel, H.; Lee, R.C.; Gehl, J. Electroporation and Cellular Physiology. In Clinical Aspects of Electroporation; Kee, S.T., Gehl, J., Lee, E.W., Eds.; Springer: New York, NY, USA, 2011; pp. 9–17. [Google Scholar]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct Therapeutic Applications of Calcium Electroporation to Effectively Induce Tumor Necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Hotta, K.; Oka, K. Spatiotemporal quantification of subcellular ATP levels in a single HeLa cell during changes in morphology. Sci. Rep. 2015, 5, 16874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, S.J.; Hosford, M.A.; Molitoris, B.A. Mechanism of Actin Polymerization in Cellular ATP Depletion. J. Boil. Chem. 2003, 279, 5194–5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, B.D.; Massiera, G.; Van Citters, K.M.; Crocker, J.C. The consensus mechanics of cultured mammalian cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10259–10264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolstykh, G.P.; Beier, H.; Roth, C.C.; Thompson, G.L.; Ibey, B.L. 600ns pulse electric field-induced phosphatidylinositol4,5-bisphosphate depletion. Bioelectrochemistry 2014, 100, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Sun, C.; Cao, Z.; Bao, N.; Xing, J.; Lu, C. Release of Intracellular Proteins by Electroporation with Preserved Cell Viability. Anal. Chem. 2012, 84, 8102–8105. [Google Scholar] [CrossRef]
- Rosazza, C.; Escoffre, J.M.; Zumbusch, A.; Rols, M.-P. The Actin Cytoskeleton Has an Active Role in the Electrotransfer of Plasmid DNA in Mammalian Cells. Mol. Ther. 2011, 19, 913–921. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell–Cell Junctions. Curr. Boil. 2018, 28, R445–R457. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Boil. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Derm. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [Green Version]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomova, O.; Gianulis, E.; Pakhomov, A.G. Different cell sensitivity to pulsed electric field. In Handbook of Electroporation; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 337–352. [Google Scholar]
- Bonakdar, M.; Graybill, P.M.; Davalos, R.V. A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC Adv. 2017, 7, 42811–42818. [Google Scholar] [CrossRef] [PubMed]
- Markelc, B.; Bellard, E.; Sersa, G.; Jesenko, T.; Pélofy, S.; Teissie, J.; Frangež, R.; Rols, M.-P.; Cemazar, M.; Golzio, M. Increased permeability of blood vessels after reversible electroporation is facilitated by alterations in endothelial cell-to-cell junctions. J. Control. Release 2018, 276, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Quintero, S.V.; Datta, A.; Amaya, R.; Elwassif, M.; Bikson, M.; Tarbell, J.M. DBS-relevant electric fields increase hydraulic conductivity ofin vitroendothelial monolayers. J. Neural Eng. 2010, 7, 16005. [Google Scholar] [CrossRef] [Green Version]
- Ghartey-Tagoe, E.B.; Morgan, J.S.; Neish, A.S.; Prausnitz, M.R. Increased permeability of intestinal epithelial monolayers mediated by electroporation. J. Control. Release 2005, 103, 177–190. [Google Scholar] [CrossRef]
- Shankayi, Z.; Firoozabadi, S.M.P.; Saraf, H.Z. The Endothelial Permeability Increased by Low Voltage and High Frequency Electroporation. J. Biomed. Phys. Eng. 2013, 3, 87–92. [Google Scholar]
- Sharabi, S.; Bresler, Y.; Ravid, O.; Shemesh, C.; Atrakchi, D.; Schnaider-Beeri, M.; Gosselet, F.; Dehouck, L.; Last, D.; Guez, D.; et al. Transient blood–brain barrier disruption is induced by low pulsed electrical fields in vitro: An analysis of permeability and trans-endothelial electric resistivity. Drug Deliv. 2019, 26, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Rems, L.; Ušaj, M.; Kandušer, M.; Rebersek, M.; Miklavcic, D.; Pucihar, G. Cell electrofusion using nanosecond electric pulses. Sci. Rep. 2013, 3, 3382. [Google Scholar] [CrossRef] [Green Version]
- Blangero, C.; Rols, M.-P.; Teissie, J. Cytoskeletal reorganization during electric-field-induced fusion of Chinese hamster ovary cells grown in monolayers. Biochim. Biophys. Acta (BBA) Biomembr. 1989, 981, 295–302. [Google Scholar] [CrossRef]
- Gerisch, G.; Ecke, M.; Neujahr, R.; Prassler, J.; Stengl, A.; Hoffmann, M.; Schwarz, U.S.; Neumann, E. Membrane and actin reorganization in electropulse-induced cell fusion. J. Cell Sci. 2013, 126, 2069–2078. [Google Scholar] [CrossRef] [Green Version]
- Bogatcheva, N.V.; Verin, A.D. Reprint of “The role of cytoskeleton in the regulation of vascular endothelial barrier function” [Microvascular Research 76 (2008) 202–207]. Microvasc. Res. 2009, 77, 64–69. [Google Scholar] [CrossRef]
- Alieva, I.B.; Zemskov, E.A.; Smurova, K.M.; Kaverina, I.N.; Verin, A.D. The leading role of microtubules in endothelial barrier dysfunction: Disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J. Cell. Biochem. 2013, 114, 2258–2272. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Smurova, K.; Birukova, A.A.; Usatyuk, P.; Liu, F.; Kaibuchi, K.; Ricks-Cord, A.; Natarajan, V.; Alieva, I.; Garcia, J.G.; et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J. Cell. Physiol. 2004, 201, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.A.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood–brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Prasain, N.; Stevens, T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc. Res. 2008, 77, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayless, K.J.; Davis, G.E. Microtubule depolymerization rapidly collapses capillary tube networks in vitro and angiogenic vessels in vivo through the small GTPase Rho. J. Biol. Chem. 2004, 279, 11686–11695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon-Sanabria, V.; Pohler, S.; Eswar, V.J.; Bierowski, M.; Gomez, E.W.; Dong, C. VE-Cadherin Disassembly and Cell Contractility in the Endothelium are Necessary for Barrier Disruption Induced by Tumor Cells. Sci. Rep. 2017, 7, 45835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.Y.; Shen, Q.; Wu, M.H. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet. 2009, 2009, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markelc, B.; Cemazar, M.; Sersa, G. Effects of Reversible and Irreversible Electroporation on Endothelial Cells and Tissue Blood Flow. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 607–620. [Google Scholar]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer. Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef]
- Cemazar, M.; Parkins, C.S.; Holder, A.L.; Chaplin, D.J.; Tozer, G.M.; Sersa, G. Electroporation of human microvascular endothelial cells: Evidence for an anti-vascular mechanism of electrochemotherapy. Br. J. Cancer 2001, 84, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajšek, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br. J. Cancer 2008, 98, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Chen, X.; Pakhomov, A.G.; Baldwin, W.H.; Sheikh, S.; Pomicter, J.L.; Ren, W.; Osgood, C.; Swanson, R.J.; Kolb, J.F. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int. J. Cancer 2009, 125, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardet, S.M.; Carr, L.; Soueid, M.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R. Multiphoton imaging reveals that nanosecond pulsed electric fields collapse tumor and normal vascular perfusion in human glioblastoma xenografts. Sci. Rep. 2016, 6, 34443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Tran, K.; Sheikh, S.; Athos, B.; Kreis, M.; Nuccitelli, P. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int. J. Cancer 2010, 127, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Swanson, R.J.; Kolb, J.F.; Nuccitelli, R.; Schoenbach, K.H. Histopathology of normal skin and melanomas after nanosecond pulsed electric field treatment. Melanoma Res. 2009, 19, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, E. Getting cancer drugs into the brain. Nature 2018, 561, S46–S47. [Google Scholar] [CrossRef]
- De Vries, N.A.; Beijnen, J.H.; Boogerd, W.; Van Tellingen, O. Blood–brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev. Neurother. 2006, 6, 1199–1209. [Google Scholar] [CrossRef]
- Lorenzo, M.F.; Thomas, S.; Kani, Y.; Hinckley, J.; Lee, M.; Adler, J.; Verbridge, S.S.; Hsu, F.-C.; Robertson, J.; Davalos, R.V.; et al. Temporal Characterization of Blood-Brain Barrier Disruption with High-Frequency Electroporation. Cancers 2019, 11, 1850. [Google Scholar] [CrossRef] [Green Version]
- Sharabi, S.; Last, D.; Guez, D.; Daniels, D.; Hjouj, M.I.; Salomon, S.; Maor, E.; Mardor, Y. Dynamic effects of point source electroporation on the rat brain tissue. Bioelectrochemistry 2014, 99, 30–39. [Google Scholar] [CrossRef]
- Arena, C.; Garcia, P.A.; Sano, M.; Olson, J.D.; Rogers-Cotrone, T.; Rossmeisl, J.H.; Davalos, R.V. Focal blood-brain-barrier disruption with high-frequency pulsed electric fields. Technology 2014, 2, 206–213. [Google Scholar] [CrossRef]
- Hjouj, M.; Last, D.; Guez, D.; Daniels, D.; Sharabi, S.; Lavee, J.; Rubinsky, B.; Mardor, Y. MRI Study on Reversible and Irreversible Electroporation Induced Blood Brain Barrier Disruption. PLoS ONE 2012, 7, e42817. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Rossmeisl, J.H.; Robertson, J.L.; Olson, J.D.; Johnson, A.J.; Ellis, T.L.; Davalos, R.V. 7.0-T Magnetic Resonance Imaging Characterization of Acute Blood-Brain-Barrier Disruption Achieved with Intracranial Irreversible Electroporation. PLoS ONE 2012, 7, e50482. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, Y.-H.; Yin, D.; Koeffler, H.P.; Sawcer, D.E.; Vernier, P.T.; Gundersen, M.A. Differential Sensitivities of Malignant and Normal Skin Cells to Nanosecond Pulsed Electric Fields. Technol. Cancer Res. Treat. 2011, 10, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frandsen, S.K.; Gehl, J. A Review on Differences in Effects on Normal and Malignant Cells and Tissues to Electroporation-Based Therapies: A Focus on Calcium Electroporation. Technol. Cancer Res. Treat. 2018, 17, 1533033818788077. [Google Scholar] [CrossRef]
- Ivey, J.W.; Latouche, E.L.; Sano, M.; Rossmeisl, J.H.; Davalos, R.V.; Verbridge, S.S. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep. 2015, 5, 17157. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, V.; Mythreye, K.; O’Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.E.; Jin, Y.-S.; Rao, J.-Y.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Havelka, D.; Kucera, O.; Deriu, M.; Cifra, M. Electro-Acoustic Behavior of the Mitotic Spindle: A Semi-Classical Coarse-Grained Model. PLoS ONE 2014, 9, e86501. [Google Scholar] [CrossRef]
- Goswami, I.; Perry, J.B.; Allen, M.E.; Brown, D.A.; Von Spakovsky, M.R.; Verbridge, S.S. Influence of Pulsed Electric Fields and Mitochondria-Cytoskeleton Interactions on Cell Respiration. Biophys. J. 2018, 114, 2951–2964. [Google Scholar] [CrossRef] [Green Version]




| Study (Year) | Cell Type (A: adherent; S: suspension; M: monolayer) | Pulse Length | Field Strength (kV/cm) | Pulse # (freq) | Pulsation Buffer (with(+) or without(−) Ca+2) | Cytoskeletal Agents | Focus | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Harkin et al. (1996) [68] | Chick embryo corneal fibroblasts (A) | 10–20 ms 1 | 0.5, 0.625, 0.75, 0.875, 1.0 | 1 (N/A) | Basal Media (+ Ca+2); Buffers (+/− Ca+2) | Actin MT IF | Media as pulsation buffer inhibited migration for 2 h, caused MT loss after 10 min, but showed MT recovery in 3–4 h; Some buffers preserved migration and MTs, excepted with high concentrations of CaCl. Extracellular calcium adversely affects cell migration due to MT disruption. Staining showed no impact to actin. Perinuclear collapse of IFs, with recovery in 3–4 h. | |
| Chopinet et al. (2013) [59] | CHO wild type (A) | 5 ms | 0.4 | 8 (1 Hz) | Buffer (− Ca+2) | Actin | AFM measurements showed YM decreased 40% after PEFs; YM more spatially homogeneous within 1 min; YM similar for electrode-facing regions and perpendicular-facing regions; Membrane rippling, loss of actin fibers 3–15 min; YM not correlated with cell resealing time; Cell swelling present; Cells re-spread by 23 min. | |
| Chopinet et al. (2014) [69] | CHO wild type (A) | 5 ms | 0.4 | 8 (1 Hz) | Buffer (− Ca+2) | LatB LatA | Actin | AFM showed YM of CHO cells decreased 30% by LatB, and recovered in 35 min after drug removal; Magnitude and duration of YM response are similar between PEF treatment and recovery from LatB; Cells do not recover from LatA and PEFs. PEFs before LatB treatment showed additive effects. |
| Hohenberger et al. (2011) [70] | BY-2 2 (S) | 3 ms; 10 ns | 0.8, 1.6; 33 | 1–10, (1 Hz); 1–20, (NR) | Buffers (+/− Ca+2) | Actin MT | Genetically modified BY-2 cells with increased actin bundling showed less PI uptake; Actin bundling stabilized the cell membrane against permeabilization after msPEFs and nsPEFs. | |
| Downey et al. (1990) [71] | Human Neutrophils (S) | NR 3 | NR | 2 (NR) | Buffer (+ CA+2) | Actin | Influx of extracellular calcium post-PEFs caused depolymerization of f-actin. | |
| Perrier et al. (2019) [72] | Actin-GUV; Empty-GUV | 500 µs | 0.1–3, 3–10 | 1–30, 2–4 (0.017 Hz) | Buffer (− Ca+2) | Actin | Actin-GUVs had increased and prolonged dye uptake compared to empty-GUVs; Actin-GUVs had reduced electrodeformation; Actin cortex fluorescence decreased after PEFs; Electrophoretic effects on actin calculated to be 4× greater than electrodeformation effects. | |
| Rols et al. (1991) [63] | CHO-WTT (A) | 100 µs | 1.5 | 10 (1 Hz) | Buffer (− Ca+2) | CytB, COL, ATP, GTP | Actin MT | Pretreatment with COL decreased resealing time and electrofusion rate post-PEFs; CytB had no significant change on electrofusion rates. |
| Rols et al. (1992) [61] | CHO-WTT (A) RBC (S) | 100 µs | 1.8, 2.4 | 10 (1 Hz) | Buffer (− Ca+2) | CytB, COL, ATP, GTP | Actin MT | COL-treated cells resealed 3× faster; ATP/GTP in buffer did not affect resealing time; Pore resealing, but not pore formation, affected by cytoskeleton; Microvilli density increased post-PEFs. |
| Teissie et al. (1994) [62] | CHO-WTT (A) RBC (S) | 100 µs | 1.8, 2.4 | 10 (1 Hz) | Buffer (− Ca+2) | CytB, COL, ATP, GTP | Actin MT | COL-treated cells resealed 3x faster; Microvilli density increased post-PEFs; Extracellular ATP increased microvilli length; Resealing rate was dependent on MTs. |
| Kanthou et al. (2006) [60] | HUVEC (M) | 100 µs | 0.05, 0.1, 0.15, 0.2 | 3 (1 Hz) | Basal Media (+ Ca+2) | Actin MT IF | Actin and MTs depolymerized in 5 min; Actin became honeycomb-like; MTs fragmented; Burst of pMLC at 30 and 60 min; Cytoskeletal recovery 1–2 h; IFs relatively unchanged, except at cell periphery. | |
| Meulenberg et al. (2012) [53] | HMEC-1 (M) | 100 µs | 0.068, 0.137, 0.274, 0.411, 0.548, 0.685 | 8 (1 Hz) | Buffer (− Ca+2) | Actin MT | Actin stress fibers thinned, fragmented, and took on a honeycomb-like organization; ECT caused cell shrinkage; MTs became densely packed, less extended, and fragmented; Partial monolayer recovery at 24 h for PEFs, but no recovery for ECT; Cell swelling by 10 min; Cell edges ruffled at 2 h; ECT caused more rapid increase in membrane permeability. | |
| Szewczyk et al. (2018) [73] | C2C12 (A, S) RD (A, S) | 100 µs | 0.6, 0.8, 1 | 8 (1 Hz) | Buffers (+/− Ca+2) | Actin | Ca+2 in buffer increased zyxin expression and actin stress fiber tension in normal C2C12 cells, but decreased zyxin expression and depolymerized actin in cancerous RD cells; Zyxin changes indicated altered cell–cell and cell–substrate connections; Adherent cells showed higher viability after PEFs than suspended cells. | |
| Kim et al. (2020) [74] | NCI-H640 (A) MCR-5 (A) | 100 µs | 0.3, 0.5, 0.7, 1 | 8 (10 Hz) | Basal Media (+ Ca+2) | CytD | Actin | CytD pretreatment decreased PI uptake after PEFs compared to PEFs alone; Annexin V-FITC signal decreased with low concentrations of CytD and low field strengths. |
| Pehlivanova et al. (2012) [56] | MDA-MB-231 (A); MCF-7 (A); NIH/3T3 (A) | Bipolar: 50–20–50 µs | 0.2, 0.5, 1.0 | 8 (1 Hz) | Basal Media (+ Ca+2) | Actin | Adhesion post-PEFs was cell-type and field-strength dependent; More cytoskeletal disruption in cancerous cells than fibroblasts; Stress fibers were thinner, fewer, and at high fields located peripherally; Podosomes formed; Actin recovered in 24–48 h, except at high fields. | |
| Pakhomov et al. (2014) [65] | CHO-K1 (A) | 600 ns | 1.92 | 1, 4 (2 Hz) | Buffer (− Ca+2) | Actin | Mitigating cell swelling prevented actin disruption post-PEFs. Without mitigating swelling, cells showed increased fluorescence of diffuse actin, reduced bright spots, and reduced overall actin fluorescence. | |
| Thompson et al. (2014) [75] | CHO-K1 (A) | 600 ns | 16.2 | 1, 20 (NR) | Buffers (+/− Ca+2) | PTX | MT | Ca+2 in buffer caused MT disruption and halted lysosome transport; MT disruption occured despite mitigating blebbing and swelling; PTX stabilized MTs against depolymerization after PEFs. |
| Thompson et al. (2016) [76] | CHO-K1 (A) | 600 ns; 10 ns | 27.7; 150 | 1, 5, 10, 20 (1 Hz) | Buffers (+/− Ca+2) | IF | Localization of cortical lamin within the nucleus after PEFs; Disruption of lamin cortex correlated with nuclear permeabilization. | |
| Tolstykh et al. (2017) [77] | CHO-K1 (A) | 600 ns | 16.2 | 1, 20; (5 Hz) | Buffer (+ CA+2) | Actin | PIP2 depletion and PLC activity led to cell swelling and blebbing; Edelfosine to block PLC activity inhibited blebbing. | |
| Xiao et al. (2011) [78] | HepG2 (A) | 450 ns | 8 | 30 (1 Hz) | NR | CytB | Actin | CytB treatment before PEFs decreased necrotic and apoptotic cells; CytB alone did not decrease viability compared to controls. |
| Ford et al. (2010) [51] | B16-F10 (S) | 300 ns | 12, 18, 26, 40, 60 | 1, 3, 10 (NR) | Buffer (− Ca+2) | Actin | Caspase activity and cytoskeletal integrity mutually exclusive; ATP decreased after nsPEFs. | |
| Steuer et al. (2016) [57] | WB-F344 (M) | 100 ns | 15,20 | 20 (NR) | Complete Media (+ Ca+2) | Actin | F-actin fragmented, less organized, and depolymerized after PEFs; cell morphology generally unchanged; Partial actin recovery by 60 min. | |
| Steuer et al. (2017) [55] | WB-F344 (M); WB-Ras (M) 4 | 100 ns | 20 | 20 (NR) | Complete Media (+ Ca+2) | Actin | AFM showed >30% decrease in YM after 8 min; Actin fibers shorter, less aligned at 5 min; increased diffuse fluorescence at 15 min; YM recovered to control values at 13–28 min; Partial recovery 30–60 min; PEFs did not induce tumorigenic behavior. | |
| Stacey et al. (2011) [49] | Jurkat (S) HeLa (A) SV40 (A) | 60 ns | 60 | 1 (N/A) | Buffer (NR) | CytB | Actin | Adherent cells had ruffled membranes and rounded up with speckled actin spots; Jurkat cells showed actin speckling; Decreased viability in HeLa and SV40 cells after pretreatment with CytB. |
| Rassokhin et al. (2011) [67] | U-937 (A) | 60 ns | 10 | >1000 (10–20 Hz) | Buffer (− Ca+2) | CytD | Actin | Pseudopod-like bleb (PLB) growth toward the anode during PEFs; CytD prevented PLBs; Actin caused unique shape; Inhibiting cell swelling prevented PLBs; Not replicated in CHO, Jurkat, or GH3 cells. |
| Dutta et al. (2015) [64] | Jurkat Clone E6-1 (S) | 60 ns | 15, 60 | 1 (N/A) | Complete Media (+ Ca+2) | Actin | AFM showed 53% decrease in YM after 15 kV/cm PEFs and minimal actin/morphological changes; At 60 kV/cm, YM decreased 85%; Cell shape changed, peripheral actin became more diffuse, and actin foci formed. | |
| Marracino et al. (2019) [79] | N/A | 30 ns | 200, 500, 1000 | 1 (N/A) | N/A | MT | MD simulations showed tubulin dipole moment increased 50% at 200 kV/cm and 300% at 1 MV/cm; No unfolding of structural motifs, but C-terminus tail pulled away from tubulin body. | |
| Průša et al. (2019) [80] | N/A | 30 ns | 1000 | 1 (N/A) | N/A | MT | MD simulations of kinesin-I docked to a tubulin heterodimer indicated altered kinesin dipole properties, altered contact surface area between kinesin and tubulin, and altered structures including MT binding motifs and nucleotide hydrolysis sites. | |
| Chafai et al. (2019) [81] | N/A | 11 ns | 20 | 100, 200, 400, 800 (1 Hz) | Buffer (− Ca+2) | MT | Purified tubulin showed decreased polymerization after PEFs; Autofluorescence measurements suggested conformational changes after PEFs; Altered zeta potential of tubulin after PEFs; AFM showed altered tubulin structures after PEFs; Immunoblots showed no damage to tubulin. | |
| Havelka et al. (2019) [82] | RBL-2H3 (A) | 11 ns | ~67.5 | 4000 (100 Hz) | Buffer (+ Ca+2) | MT | GFP-tagged MT end-tracking protein EB3 showed decreased fluorescence and size after PEFs. | |
| Thomson et al. (2013) [83] | CHO-K1 (A) U-937 (S) Jurkat Clone E6-1 (S) | 10 ns | 150 | 100 (NR) | Complete Media (+ Ca+2) | PTX, JAS, LatA, NOC | Actin MT | LatA pretreatment decreased CHO-K1 elasticity to levels of Jurkat cells, however CHO-K1 cells had higher viability after PEFs; MT disruption by NOC decreased PI uptake and Annexin V-FITC fluorescence; LatA pretreatment increased PI uptake and Annexin V-FITC; JAS and PTX pretreatment did not change membrane damage after PEFs. |
| Berghöfer et al. (2009) [58] | BY-2 (S) | 10 ns | 33 | 1 (N/A) | Buffer (− Ca+2) | PHD | Actin MT | Depolymerization of cortical actin; Detachment of transvacuolar actin bundles from cell periphery; Actin contraction toward the nucleus; PHD pretreatment decreased uptake of trypan blue and suppressed actin detachment from cell periphery; MTs affected within 1 min, and maximally disordered by 3 min. |
| Thomson et al. (2014) [54] | CHO-K1 (A) | 10 ns | 150 | 50, 100 (1 Hz) | Complete Media (+ Ca+2) | LatA | Actin | AFM showed that YM of newly-adherent cells decreased ~50% after PEFs and caused partial loss of the actin cortex; LatA caused ~80% decrease in YM and fully disrupted the actin cortex; LatA treatment before PEFs increased PI uptake and decreased viability. |
| Carr et al. (2017) [47] | U-87 MG (A) | 10 ns | 44 | 100 (10 Hz) | Buffers (+/− Ca+2) | MT | MTs showed buckling, breaking, depolymerization; MT end-tracking protein EB3 showed altered dynamics post-PEFs. Decreased tubulin and EB3 comet fluorescence after PEFs; Decreased number of EB3 comets, but comet length increased; MT disruption independent of intra/extracellular calcium; MT disruption temporally linked with mitochondria depolarization. | |
| Timmons et al. (2018) [84] | N/A | 10 ns | 50–750 | 1 (N/A) | N/A | MT | MD simulations indicated conformational changes to charged and flexible regions of sidechains and loops of tubulin such as α: H1-B2 loop, β: M-loop, and c-termini. Intradimer curvature increased in simulations after PEFs. |
| Actin | Inhibit Polymerization | Stabilize Polymerization | Studies Used |
| Cytochalasin B or D (CytB/CytD) | X | [49,61,62,67,74,78] | |
| Latrunculin A or B (LatA/LatB) | X | [54,69,83] | |
| Phalloidin (PHD) | X | [58] | |
| Jasplakinolide (JAS) | X | [83] | |
| ATP | X | [61,62] | |
| Microtubules | Inhibit Polymerization | Stabilize Polymerization | Studies Used |
| Colchicine (COL) | X | [61,62] | |
| Nocodazole (NOC) | X | [83] | |
| Paclitaxel (PTX) | X | [75,63] | |
| GTP | X | [61,62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graybill, P.M.; Davalos, R.V. Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers 2020, 12, 1132. https://doi.org/10.3390/cancers12051132
Graybill PM, Davalos RV. Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers. 2020; 12(5):1132. https://doi.org/10.3390/cancers12051132
Chicago/Turabian StyleGraybill, Philip M., and Rafael V. Davalos. 2020. "Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies" Cancers 12, no. 5: 1132. https://doi.org/10.3390/cancers12051132
APA StyleGraybill, P. M., & Davalos, R. V. (2020). Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers, 12(5), 1132. https://doi.org/10.3390/cancers12051132