Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer
Abstract
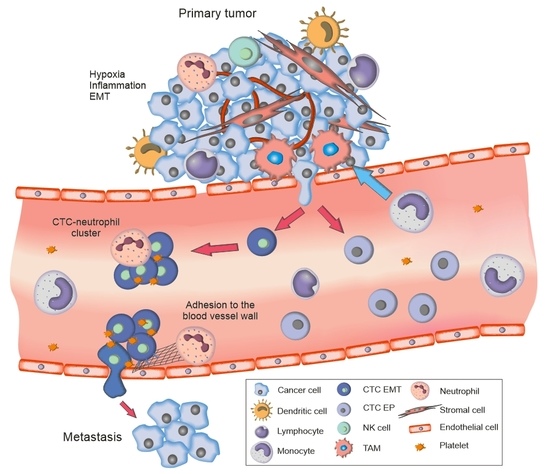
:1. Introduction
2. Results
2.1. Prognostic Role of Circulating Tumor Cells
2.2. Complete Blood Count-Derived Inflammation-Based Scores
2.3. Increased Prognostic Value of CTC EMT in Combination with Complete Blood Count-Derived Inflammation-Based Scores
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Identification of Gene Transcripts in CD45-Enriched Subsets
4.3. Complete Blood Count and CBC-Derived Inflammation-Based Scores
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Arciero, C.A.; Styblo, T.M. Clinically Established Prognostic Factors in Breast Cancer. In The Breast; Elsevier: Amsterdam, The Netherlands, 2018; pp. 250–257. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Szczerba, B.M.; Aceto, N. Circulating Tumor Cell-Neutrophil Tango along the Metastatic Process. Cancer Res. 2019, 79, 6067–6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Bonavita, E.; Barajon, I.; Garlanda, C.; Mantovani, A.; Jaillon, S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Ponzetta, A.; Carriero, R.; Carnevale, S.; Barbagallo, M.; Molgora, M.; Perucchini, C.; Magrini, E.; Gianni, F.; Kunderfranco, P.; Polentarutti, N.; et al. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell 2019, 178, 346–360. [Google Scholar] [CrossRef] [Green Version]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef] [Green Version]
- Piccard, H.; Muschel, R.J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012, 82, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef]
- Mego, M.; Cierna, Z.; Janega, P.; Karaba, M.; Minarik, G.; Benca, J.; Sedlackova, T.; Sieberova, G.; Gronesova, P.; Manasova, D.; et al. Relationship between circulating tumor cells and epithelial to mesenchymal transition in early breast cancer. BMC Cancer 2015, 15, 533. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Gao, H.; Lee, B.N.; Cohen, E.N.; Tin, S.; Giordano, A.; Wu, Q.; Liu, P.; Nieto, Y.; Champlin, R.E.; et al. Prognostic Value of EMT-Circulating Tumor Cells in Metastatic Breast Cancer Patients Undergoing High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation. J. Cancer 2012, 3, 369–380. [Google Scholar] [CrossRef]
- Mego, M.; Karaba, M.; Minarik, G.; Benca, J.; Silvia, J.; Sedlackova, T.; Manasova, D.; Kalavska, K.; Pindak, D.; Cristofanilli, M.; et al. Circulating Tumor Cells with Epithelial-to-mesenchymal Transition Phenotypes Associated With Inferior Outcomes in Primary Breast Cancer. Anticancer. Res. 2019, 39, 1829–1837. [Google Scholar] [CrossRef]
- De Giorgi, U.; Mego, M.; Scarpi, E.; Giordano, A.; Giuliano, M.; Valero, V.; Alvarez, R.H.; Ueno, N.T.; Cristofanilli, M.; Reuben, J.M. Association between circulating tumor cells and peripheral blood monocytes in metastatic breast cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866065. [Google Scholar] [CrossRef] [Green Version]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Avalos, C.; Chang, A.Y.; Dirbas, F.M.; Yim, J.H.; Waisman, J.; Lee, P.P. Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine 2020, 52, 102631. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Huang, D.H.; Chen, Z.Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381–75388. [Google Scholar] [CrossRef] [PubMed]
- Huszno, J.; Kolosza, Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol. Lett. 2019, 18, 6275–6283. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.-L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Lopez, J.; Fidalgo-Zapata, A.; Lopez-Agudelo, L.; Vasquez-Trespalacios, E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS ONE 2018, 13, e0207224. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Chen, D.; Duan, S.; Zhao, Y.; Wu, C.; Zhu, F.; Chen, C.; Chen, Y. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: A meta-analysis. Cancer Cell Int. 2018, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Wu, J.; Jia, H.; Yang, Y.; Zhang, X.; Chen, K.; Su, F. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS ONE 2015, 10, e0143061. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017, 34, 12. [Google Scholar] [CrossRef]
- Thery, L.; Meddis, A.; Cabel, L.; Proudhon, C.; Latouche, A.; Pierga, J.-Y.; Bidard, F.-C. Circulating tumor cells in early breast cancer. JNCI Cancer Spectr. 2019, 3, pkz026. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.-Y.; Taran, F.-A.; Fehm, T.; Hall, C.; De Groot, M.R.; Bidard, F.-C.; Friedl, T.W. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, P.K.; Cummins, A.G.; Price, T.J.; Roberts-Thomson, I.C.; Hardingham, J.E. Circulating tumour cells: The evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann. Oncol. 2014, 25, 1506–1516. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558–81571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wu, T.; Peng, X.; Liu, J.; Liu, F.; Wu, S.; Liu, S.; Dong, Y.; Xie, S.; Ma, S. Mesenchymal phenotype of circulating tumor cells is associated with distant metastasis in breast cancer patients. Cancer Manag. Res. 2017, 9, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Ma, F.; Li, C.; Wu, S.; Hu, S.; Huang, J.; Sun, X.; Wang, J.; Luo, Y.; Cai, R.; et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial–mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun. 2019, 39, 1. [Google Scholar] [CrossRef]
- Bulfoni, M.; Gerratana, L.; Del Ben, F.; Marzinotto, S.; Sorrentino, M.; Turetta, M.; Scoles, G.; Toffoletto, B.; Isola, M.; Beltrami, C.A. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Ye, X.; Brabletz, T.; Kang, Y.; Longmore, G.D.; Nieto, M.A.; Stanger, B.Z.; Yang, J.; Weinberg, R.A. Upholding a role for EMT in breast cancer metastasis. Nature 2017, 547, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeke, S.; Mograbi, B.; Alix-Panabières, C.; Hofman, P. Never Travel Alone: The Crosstalk of Circulating Tumor Cells and the Blood Microenvironment. Cells 2019, 8, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.; Zhao, X.; Li, X.F.; Xu, R. Neutrophil-to-Lymphocyte Ratio Predicts PSA Response and Prognosis in Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarraf, K.M.; Belcher, E.; Raevsky, E.; Nicholson, A.G.; Goldstraw, P.; Lim, E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2009, 137, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Yao, M.; Xing, C.; Wang, W.; Yao, J.; Hong, Y.; Liu, Y.; Fu, P. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Onco Targets Ther. 2016, 9, 5567–5575. [Google Scholar] [CrossRef] [Green Version]
- Suppan, C.; Bjelic-Radisic, V.; La Garde, M.; Groselj-Strele, A.; Eberhard, K.; Samonigg, H.; Loibner, H.; Dandachi, N.; Balic, M. Neutrophil/Lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer 2015, 15, 1027. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Huang, X.-Z.; Song, Y.-X.; Gao, P.; Sun, J.-X.; Wang, Z.-N. High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: A meta-analysis. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, C.M.; Madrona, A.P.; Vazquez, P.G.; Fernández, P.G.; Merino, G.R.; Romero, J.A.; Paricio, P.P. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin. Transl. Oncol. 2018, 20, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lu, X.; Liu, Q.; Zhang, T.; Li, P.; Qiao, W.; Deng, M. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019, 8, 4135–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Liu, Y.; Jin, H.; Liu, X.; Lv, K.; Wei, H.; Du, C.; Wang, S.; Wei, B.; Fu, P. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014, 7, 1743. [Google Scholar] [PubMed] [Green Version]
- Krenn-Pilko, S.; Langsenlehner, U.; Thurner, E.M.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer 2014, 110, 2524–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, S.K.; Fu, S.M.; Fu, Y.P.; Zhang, H.W. Neutrophil to lymphocyte ratio is a prognostic factor for disease free survival in patients with breast cancer underwent curative resection. Medicine (Baltimore) 2018, 97, e11898. [Google Scholar] [CrossRef] [PubMed]
- Ivars Rubio, A.; Yufera, J.C.; de la Morena, P.; Fernández Sánchez, A.; Navarro Manzano, E.; García Garre, E.; García Martinez, E.; Marín Zafra, G.; Sánchez Cánovas, M.; García Torralba, E.; et al. Neutrophil-lymphocyte ratio in metastatic breast cancer is not an independent predictor of survival, but depends on other variables. Sci. Rep. 2019, 9, 16979. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Ye, F.; Huang, X.; Li, S.; Yang, L.; Xiao, X.; Xie, X. Prognostic Significance of Preoperative Circulating Monocyte Count in Patients with Breast Cancer: Based on a Large Cohort Study. Medicine (Baltimore) 2015, 94, e2266. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Wculek, S.K.; Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B.; Klameth, L.; Hochmair, M.J. Small cell lung cancer: Recruitment of macrophages by circulating tumor cells. Oncoimmunology 2016, 5, e1093277. [Google Scholar] [CrossRef] [Green Version]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.F.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2016, 2, 15025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Z.; Wang, C.; Ye, Z.; Rossi, G.; Sun, C.; Li, L.; Zhu, Z.; Yang, H.; Cristofanilli, M. Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer. Breast Cancer Res. Treat. 2017, 165, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Reduzzi, C.; Vismara, M.; Gerratana, L.; Silvestri, M.; De Braud, F.; Raspagliesi, F.; Verzoni, E.; Di Cosimo, S.; Locati, L.D.; Cristofanilli, M.; et al. The curious phenomenon of dual-positive circulating cells: Longtime overlooked tumor cells. Semin. Cancer Biol. 2020, 60, 344–350. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [Green Version]
- Cierna, Z.; Mego, M.; Janega, P.; Karaba, M.; Minarik, G.; Benca, J.; Sedlácková, T.; Cingelova, S.; Gronesova, P.; Manasova, D.; et al. Matrix metalloproteinase 1 and circulating tumor cells in early breast cancer. BMC Cancer 2014, 14, 472. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Mani, S.A.; Lee, B.N.; Li, C.; Evans, K.W.; Cohen, E.N.; Gao, H.; Jackson, S.A.; Giordano, A.; Hortobagyi, G.N.; et al. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: The effect of neoadjuvant therapy. Int. J. Cancer 2012, 130, 808–816. [Google Scholar] [CrossRef]




| Variables | Categories | All Patients (n = 284) | CTC EMT Negative (n = 235) | CTC EMT Positive (n = 49) | p# |
|---|---|---|---|---|---|
| Age (years) Median (range) | 59.1 (24.66–83.49) | 58.8 (24.7–83.36) | 59.5 (33.5–83.49) | 0.323 | |
| Age (years) | ≤50 | 75 (26.4) | 67 (28.5) | 8 (16.3) | 0.078 |
| >50 | 209 (73.6) | 168 (71.5) | 41 (83.7) | ||
| T-stage | T1 | 200 (70.4) | 168 (71.5) | 32 (65.3) | 0.388 |
| T2 and more | 84 (29.6) | 67 (28.5) | 17 (34.7) | ||
| Histology | IDC | 242 (85.2) | 199 (84.7) | 43 (87.8) | 0.581 |
| Others | 42 (14.8) | 36 (15.3) | 6 (12.2) | ||
| Grade | Low and intermediate | 194 (69.8) | 164 (70.4) | 30 (66.7) | 0.619 |
| High | 84 (29.6) | 69 (29.6) | 15 (33.3) | ||
| N-stage | N0 | 180 (64.3) | 151 (64.8) | 29 (61.7) | 0.685 |
| N+ | 100 (35.7) | 82 (35.2) | 18 (38.3) | ||
| LVI | Absent | 234 (82.4) | 196 (83.4) | 38 (77.6) | 0.328 |
| Present | 50 (17.6) | 39 (16.6) | 11 (22.4) | ||
| HR status $ | Negative | 46 (16.2) | 37 (15.7) | 9 (18.4) | 0.650 |
| Positive | 238 (83.8) | 198 (84.3) | 40 (81.6) | ||
| HER2 status | Negative | 240 (84.5) | 200 (85.1) | 40 (81.6) | 0.541 |
| Amplified | 44 (15.5) | 35 (14.9) | 9 (18.4) | ||
| p53 | Negative | 178 (62.9) | 144 (61.5) | 34 (69.4) | 0.301 |
| Positive | 105 (37.1) | 90 (38.5) | 15 (30.6) | ||
| bcl2 | Negative | 82 (28.9) | 64 (27.2) | 18 (36.7) | 0.182 |
| Positive | 202 (71.1) | 171 (72.8) | 31 (63.3) | ||
| Ki-67 | <20% | 174 (61.5) | 148 (63.2) | 26 (53.1) | 0.183 |
| >20% | 109 (38.5) | 86 (36.8) | 23 (46.9) | ||
| Tumor subtypes | Luminal A | 150 (53.0) | 127 (54.3) | 23 (46.9) | 0.752 |
| Luminal B | 58 (20.5) | 46 (19.7) | 12 (24.5) | ||
| HER2-positive | 44 (15.5) | 35 (15.0) | 9 (18.4) | ||
| Triple-negative | 31 (11.0) | 26 (11.1) | 5 (10.2) | ||
| NLR | <3 | 231 (84.0) | 190 (84.1) | 41 (83.7) | 0.945 |
| ≥3 | 44 (16.0) | 36 (15.9) | 8 (16.3) | ||
| MLR | <0.34 | (210) 84.3 | 172 (84.7) | 38 (82.6) | 0.721 |
| ≥0.34 | (39) 15.7 | 31 (15.3) | 8 (17.4) | ||
| PLR | <210 | 223 (84.2) | 183 (84.3) | 40(83.3) | 0.846 |
| ≥210 | 42 (15.8) | 34 (15.7) | 8 (16.7) | ||
| SII | <836 | 221 (77.8) | 180 (82.9) | 41 (85.4) | 0.678 |
| ≥836 | 44 (15.5) | 37 (17.1) | 7 (14.6) |
| Variables | HR | 95% CI | p |
|---|---|---|---|
| NLR ≥ 3 | 2.45 | 1.17–5.12 | 0.017 |
| Age > 50 | 3.15 | 1.11–8.96 | 0.031 |
| High grade | 2.36 | 1.12–4.96 | 0.024 |
| N+ | 2.18 | 1.11–4.28 | 0.023 |
| Ki-67 > 20% | 3.03 | 1.38–6.65 | 0.006 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| CTC EMT-negative, NLR < 3 | 0.006 | ||
| CTC EMT-negative, NLR ≥ 3 | 2.30 | 0.95–5.57 | 0.065 |
| CTC EMT-positive, NLR < 3 | 2.06 | 0.87–4.85 | 0.099 |
| CTC EMT-positive, NLR ≥ 3 | 8.60 | 2.35–31.48 | 0.001 |
| Age > 50 | 2.90 | 1.01–8.36 | 0.049 |
| High grade | 2.58 | 1.20–5.53 | 0.015 |
| N+ | 2.46 | 1.24–4.87 | 0.010 |
| Ki67 > 20 | 2.79 | 1.27–6.11 | 0.010 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| CTC EMT-negative, MLR < 0.34 | <0.001 | ||
| CTC EMT-negative, MLR ≥ 0.34 | 1.00 | 0.33–2.98 | 0.997 |
| CTC EMT-positive, MLR < 0.34 | 1.12 | 0.33–3.84 | 0.852 |
| CTC EMT-positive, MLR ≥ 0.34 | 13.14 | 4.35–39.67 | <0.001 |
| Age > 50 | 3.35 | 1.00–11.22 | 0.050 |
| T2 and more | 0.41 | 0.17–0.97 | 0.043 |
| High grade | 3.93 | 1.85–8.34 | <0.001 |
| N+ | 3.36 | 1.56–7.22 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklikova, S.; Minarik, G.; Sedlackova, T.; Plava, J.; Cihova, M.; Jurisova, S.; Kalavska, K.; Karaba, M.; Benca, J.; Smolkova, B.; et al. Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers 2020, 12, 1134. https://doi.org/10.3390/cancers12051134
Miklikova S, Minarik G, Sedlackova T, Plava J, Cihova M, Jurisova S, Kalavska K, Karaba M, Benca J, Smolkova B, et al. Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers. 2020; 12(5):1134. https://doi.org/10.3390/cancers12051134
Chicago/Turabian StyleMiklikova, Svetlana, Gabriel Minarik, Tatiana Sedlackova, Jana Plava, Marina Cihova, Silvia Jurisova, Katarina Kalavska, Marian Karaba, Juraj Benca, Bozena Smolkova, and et al. 2020. "Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer" Cancers 12, no. 5: 1134. https://doi.org/10.3390/cancers12051134
APA StyleMiklikova, S., Minarik, G., Sedlackova, T., Plava, J., Cihova, M., Jurisova, S., Kalavska, K., Karaba, M., Benca, J., Smolkova, B., & Mego, M. (2020). Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers, 12(5), 1134. https://doi.org/10.3390/cancers12051134