Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation
Abstract
:1. Introduction
2. Results
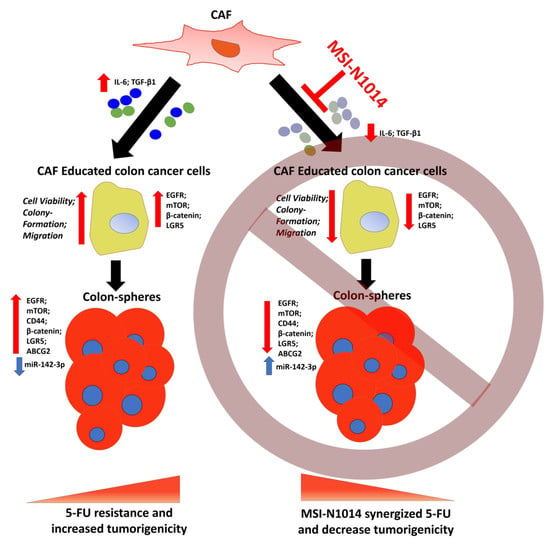
2.1. CAFs Increased Oncogenic Properties of CRC Cells with Increased Association of EGFR
2.2. MSI-N1014 Treatment Suppressed CRC Tumorigenesis
2.3. MSI-N1014 Treatments Lowered CRC’s Ability to Generate CAFs
2.4. MSI-N1014’s Anti-CRC Function Was Associated with the Induction of miR-142-3p and Reductions of Its Oncogenic Targets
2.5. MSI-N1014 Treatment Increased 5-FU Efficacy In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Colon Tumor Sphere-Formation Assay
4.3. Cell Viability Assay
4.4. Co-Culture and Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Immunofluorescence Imaging
4.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
4.7. Transient microRNA (miRNA) Transfection
4.8. Drug Combination Index (CI) Evaluation
4.9. RNA Isolation and Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Wound-Healing Migration Assay
4.11. Colony-Formation Assay
4.12. Flow Cytometry
4.13. In Vivo Evaluation of MSI-N1014
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boyle, P.; Langman, J.S. ABC of colorectal cancer: Epidemiology. BMJ 2000, 321, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Sakai, R. Direct Interaction between Carcinoma Cells and Cancer Associated Fibroblasts for the Regulation of Cancer Invasion. Cancers 2015, 7, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.T.; Beswick, E.J.; Coronado, Y.A.; Johnson, P.; O’Connell, M.R.; Watts, T.; Singh, P.; Qiu, S.; Morris, K.; Powell, D.W.; et al. CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int. J. Cancer 2016, 138, 1971–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, M.I.; Huang, J.; Hung, J.S.; Lin, Y.C.; Huang, M.J.; Lai, H.S.; Hsu, W.M.; Liang, J.T.; Huang, M.C. Beta1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget 2014, 5, 3673–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Sharma, A.; Lin, Y.; Wu, Y.; He, Q.; Gu, Y.; Xu, Z.P.; Monteiro, M.; Gu, W. Insluin and epithelial growth factor (EGF) promote programmed death ligand 1(PD-L1) production and transport in colon cancer stem cells. BMC Cancer 2019, 19, 153. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.; Kwon, C.I.; Kim, J.W.; Park, P.W.; Hahm, K.B. TWIST1 and SNAI1 as markers of poor prognosis in human colorectal cancer are associated with the expression of ALDH1 and TGF-beta1. Oncol. Rep. 2014, 31, 1380–1388. [Google Scholar] [CrossRef] [Green Version]
- Lievre, A.; Blons, H.; Laurent-Puig, P. Oncogenic mutations as predictive factors in colorectal cancer. Oncogene 2010, 29, 3033–3043. [Google Scholar] [CrossRef] [Green Version]
- Botrel, T.E.A.; Clark, L.G.O.; Paladini, L.; Clark, O.A.C. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2016, 16, 677. [Google Scholar] [CrossRef] [Green Version]
- Hecht, J.R.; Mitchell, E.P.; Yoshino, T.; Welslau, M.; Lin, X.; Chow Maneval, E.; Paolini, J.; Lechuga, M.J.; Kretzschmar, A. 5-Fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus sunitinib or bevacizumab as first-line treatment for metastatic colorectal cancer: A randomized Phase IIb study. Cancer Manag. Res. 2015, 7, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, A.B.; Zhao, F.; Meropol, N.J.; Flynn, P.J.; Wagner, L.I.; Sloan, J.; Diasio, R.B.; Mitchell, E.P.; Catalano, P.; Giantonio, B.J.; et al. Intergroup Randomized Phase III Study of Postoperative Oxaliplatin, 5-Fluorouracil, and Leucovorin Versus Oxaliplatin, 5-Fluorouracil, Leucovorin, and Bevacizumab for Patients with Stage II or III Rectal Cancer Receiving Preoperative Chemoradiation: A Trial of the ECOG-ACRIN Research Group (E5204). Oncologist 2020, 25, e798–e807. [Google Scholar] [PubMed] [Green Version]
- Chen, T.C.; Wu, C.L.; Lee, C.C.; Chen, C.L.; Yu, D.S.; Huang, H.S. Structure-based hybridization, synthesis and biological evaluation of novel tetracyclic heterocyclic azathioxanthone analogues as potential antitumor agents. Eur. J. Med. Chem. 2015, 103, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Poutahidis, T.; Erdman, S.E.; Kirsch, R.; Riddell, R.H.; Diamandis, E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 2012, 10, 1403–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strubberg, A.M.; Madison, B.B. MicroRNAs in the etiology of colorectal cancer: Pathways and clinical implications. Dis. Model. Mech. 2017, 10, 197–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, R.; Mosakhani, N.; Asadi, J.; Nouraee, N.; Mowla, S.J.; Yazdani, Y.; Mohamadkhani, A.; Poustchi, H.; Knuutila, S.; Malekzadeh, R. Downregulation of Plasma MiR-142-3p and MiR-26a-5p in Patients with Colorectal Carcinoma. Iran J. Cancer Prev. 2015, 8, e2329. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.J.; Deane, N.G.; Wu, F.; Merchant, N.B.; Zhang, B.; Jiang, A.; Lu, P.; Johnson, J.C.; Schmidt, C.; Bailey, C.E.; et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 2010, 138, 958–968. [Google Scholar] [CrossRef] [Green Version]
- Freeman, T.J.; Smith, J.J.; Chen, X.; Washington, M.K.; Roland, J.T.; Means, A.L.; Eschrich, S.A.; Yeatman, T.J.; Deane, N.G.; Beauchamp, R.D. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of beta-catenin. Gastroenterology 2012, 142, 562–571.e2. [Google Scholar] [CrossRef] [Green Version]
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979. [Google Scholar] [CrossRef] [Green Version]
- Goncalves-Ribeiro, S.; Diaz-Maroto, N.G.; Berdiel-Acer, M.; Soriano, A.; Guardiola, J.; Martinez-Villacampa, M.; Salazar, R.; Capella, G.; Villanueva, A.; Martinez-Balibrea, E.; et al. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget 2016, 7, 59766–59780. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, T.P.; Stuart, M.; Oosting, J.; Tollenaar, R.; Sier, C.F.M.; Mesker, W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer 2019, 19, 284. [Google Scholar] [CrossRef] [Green Version]
- Osawa, H.; Takahashi, H.; Nishimura, J.; Ohta, K.; Haraguchi, N.; Hata, T.; Yamamoto, H.; Mizushima, T.; Takemasa, I.; Doki, Y.; et al. Full-length LGR5-positive cells have chemoresistant characteristics in colorectal cancer. Br. J. Cancer 2016, 114, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi-Baig, K.; Ullmann, P.; Rodriguez, F.; Frasquilho, S.; Nazarov, P.V.; Haan, S.; Letellier, E. What Do We Learn from Spheroid Culture Systems? Insights from Tumorspheres Derived from Primary Colon Cancer Tissue. PLoS ONE 2016, 11, e0146052. [Google Scholar] [CrossRef] [PubMed]
- Glinka, A.; Dolde, C.; Kirsch, N.; Huang, Y.L.; Kazanskaya, O.; Ingelfinger, D.; Boutros, M.; Cruciat, C.M.; Niehrs, C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011, 12, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lau, W.; Barker, N.; Low, T.Y.; Koo, B.K.; Li, V.S.; Teunissen, H.; Kujala, P.; Haegebarth, A.; Peters, P.J.; van de Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297. [Google Scholar] [CrossRef] [PubMed]
- De Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014, 28, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.S.; Lin, X.K.; Mei, Y.; Ahmad, S.; Yan, C.X.; Jin, H.L.; Yu, H.; Chen, C.; Lin, C.Z.; Yu, J.R. Regulatory T Cells Promote Overexpression of Lgr5 on Gastric Cancer Cells via TGF-beta1 and Confer Poor Prognosis in Gastric Cancer. Front. Immunol. 2019, 10, 1741. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Feng, J.; Xie, J.; Mei, Z.; Shi, T.; Wang, S.; Du, Y.; Yang, G.; Wu, Y.; Cheng, X.; et al. Targeted blockade of TGF-beta and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci. Rep. 2017, 7, 8660. [Google Scholar] [CrossRef]
- Bu, L.; Baba, H.; Yoshida, N.; Miyake, K.; Yasuda, T.; Uchihara, T.; Tan, P.; Ishimoto, T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 2019, 38, 4887–4901. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, N.; Hara, M.; Shiga, K.; Yanagita, T.; Takasu, K.; Nakai, N.; Maeda, Y.; Hirokawa, T.; Takahashi, H.; Ishiguro, H.; et al. Eicosapentaenoic acid suppresses angiogenesis via reducing secretion of IL6 and VEGF from colon cancerassociated fibroblasts. Oncol. Rep. 2019, 42, 339–349. [Google Scholar] [PubMed]
- Troschel, F.M.; Bohly, N.; Borrmann, K.; Braun, T.; Schwickert, A.; Kiesel, L.; Eich, H.T.; Gotte, M.; Greve, B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumour. Biol. 2018, 40, 1010428318791887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; He, B.S.; Pan, B.; Pan, Y.Q.; Sun, H.L.; Liu, X.X.; Xu, X.N.; Chen, X.X.; Zeng, K.X.; Xu, M.; et al. MiR-142-3p functions as a tumor suppressor by targeting RAC1/PAK1 pathway in breast cancer. J. Cell. Physiol. 2020, 235, 4928–4940. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Dong, J.; Zhao, Y.; Peng, X.; Tang, J.; Chen, X.; Wang, L.; Hu, D.N.; Reinach, P.S.; Qu, J.; et al. miR-142-3p suppresses uveal melanoma by targeting CDC25C, TGFβR1, GNAQ, WASL, and RAC1. Cancer Manag. Res. 2019, 11, 4729–4742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Pang, D.; Yu, S. Serum level of miR-142-3p predicts prognostic outcome for colorectal cancer following curative resection. J. Int. Med. Res. 2019, 47, 2116–2125. [Google Scholar] [CrossRef] [Green Version]
- Dotse, E.; Bian, Y. Isolation of colorectal cancer stem-like cells. Cytotechnology 2016, 68, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, L.; Larson, B. Performance of a Label-Free Image-Based 2D Scratch Wound Healing Assay to Monitor Cell Migration and Its Inhibition. Available online: https://www.biotek.com/resources/application-notes/performance-of-a-label-free-image-based-2d-scratch-wound-healing-assay-to-monitor-cell-migration-and-its-inhibition/ (accessed on 5 May 2020).
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Yeh, C.T.; Wu, A.T.; Chang, P.M.; Chen, K.Y.; Yang, C.N.; Yang, S.C.; Ho, C.C.; Chen, C.C.; Kuo, Y.L.; Lee, P.Y.; et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am. J. Respir. Crit. Care Med. 2012, 186, 1180–1188. [Google Scholar] [CrossRef]





| Target | Dilution | Company and Catalog No. | Predicted MW (kDa) |
|---|---|---|---|
| GAPDH | 1:1000 | Proteintech, IL6 Rabbit mAb, 10494-1-AP | 36 |
| β-catenin | 1:1000 | Cell Signaling, β–Catenin (6B3) Rabbit mAb, #9582 | 92 |
| mTOR | 1:1000 | Cell Signaling, mTOR (7C10) Rabbit mAb, #2983 | 289 |
| LRG-5 | 1:1000 | Epitomics, LRG5, Rabbit mAb, #2495-1 | 99.9 |
| IL-6 | 1:500 | Proteintech, IL6 Rabbit mAb, 21865-1-AP | 24 |
| EGFR | 1:1000 | Proteintech, EGFR Rabbit mAb, 18986-1-AP | 165–145 |
| ABCG2 | 1:2000 | Proteintech, ABCG2 Rabbit mAb, 10051-1-AP | 60–70 |
| TGF-β1 | 1:1000 | Proteintech, TGF-β1 Rabbit mAb, 18978-1-AP | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.K.; Huang, Y.-J.; George, T.A.; Wei, P.-L.; Sumitra, M.r.; Ho, C.-L.; Chang, T.-H.; Wu, A.T.H.; Huang, H.-S. Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation. Cancers 2020, 12, 1590. https://doi.org/10.3390/cancers12061590
Yadav VK, Huang Y-J, George TA, Wei P-L, Sumitra Mr, Ho C-L, Chang T-H, Wu ATH, Huang H-S. Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation. Cancers. 2020; 12(6):1590. https://doi.org/10.3390/cancers12061590
Chicago/Turabian StyleYadav, Vijesh Kumar, Yan-Jiun Huang, Thomashire Anita George, Po-Li Wei, Maryam rachmawati Sumitra, Ching-Liang Ho, Tzu-Hao Chang, Alexander T. H. Wu, and Hsu-Shan Huang. 2020. "Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation" Cancers 12, no. 6: 1590. https://doi.org/10.3390/cancers12061590
APA StyleYadav, V. K., Huang, Y. -J., George, T. A., Wei, P. -L., Sumitra, M. r., Ho, C. -L., Chang, T. -H., Wu, A. T. H., & Huang, H. -S. (2020). Preclinical Evaluation of the Novel Small-Molecule MSI-N1014 for Treating Drug-Resistant Colon Cancer via the LGR5/β-catenin/miR-142-3p Network and Reducing Cancer-Associated Fibroblast Transformation. Cancers, 12(6), 1590. https://doi.org/10.3390/cancers12061590